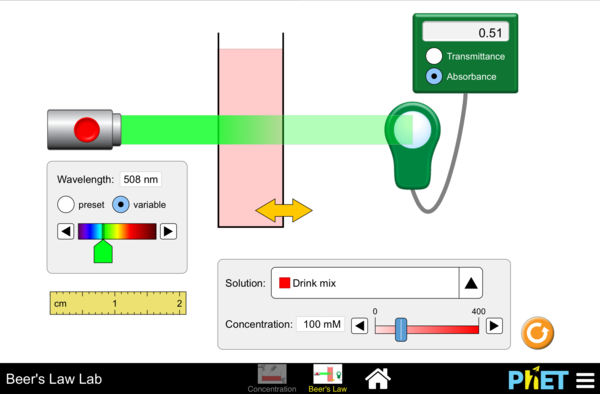
Use the link below to launch a simulation where you can alter the properties involved in spectrophotometry and examine the Beer-Lambert Law. Increase and decrease the concentration slider in the simulation:
- What happens to the contents in the cuvette?
- How does this change the Transmittance and Absorbance readings?
- Click “variable” and use the slider. What happens to the readings when the Wavelength of the laser is a similar color as the solution in the cuvette?
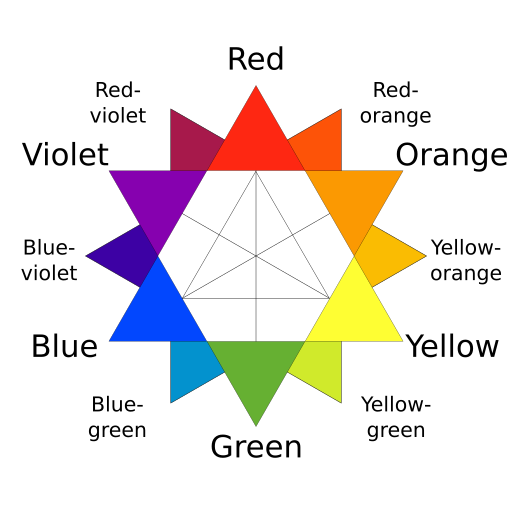
- Consult the color star below and find the color wavelength that is opposite of the color of the solution. Set the laser to this color using “variable” and the slider.
- What is the effect on Transmittance and Absorbance with this color?
- Using the previous observations (using variable wavelength slider), how would you use the relationship on Transmittance/Absorbance to best measure the concentration of a solution?
Tags: visual communication