Contents [hide]
Analytical Chemistry

Analytical chemistry plays a crucial role in various fields, including food science, environmental monitoring, pharmaceuticals, and forensics. It involves the identification, separation, and quantification of chemical substances. In the food industry, analytical chemistry techniques are essential for quality control, ensuring food safety, and complying with regulatory standards.

Natural dyes, like cochineal, create vibrant red colors used for centuries. Cochineal is a natural dye extracted from the dried bodies of an insect of the species Dactylopius coccus. This dye can be found in food and cosmetics, but the laborious nature of harvesting these insects lead to the usage of artificial chemicals for coloring instead.

The Food and Drug Administration (FDA) regulates color additives labeled as Food, Drug, and Cosmetic (FD&C) dyes. Erythrosine, also known as FD&C Red Dye #3, has been linked to potential health risks and banned from cosmetics since 1990. In January of 2025, the FDA finally ruled to ban this chemical in food due to the association of thyroid tumor induction in rats and some association with behavioral developmental issues in children. Enforcement of this ban poses a clear example of how analytical chemistry can be applied to ensure food safety.
Chromatography
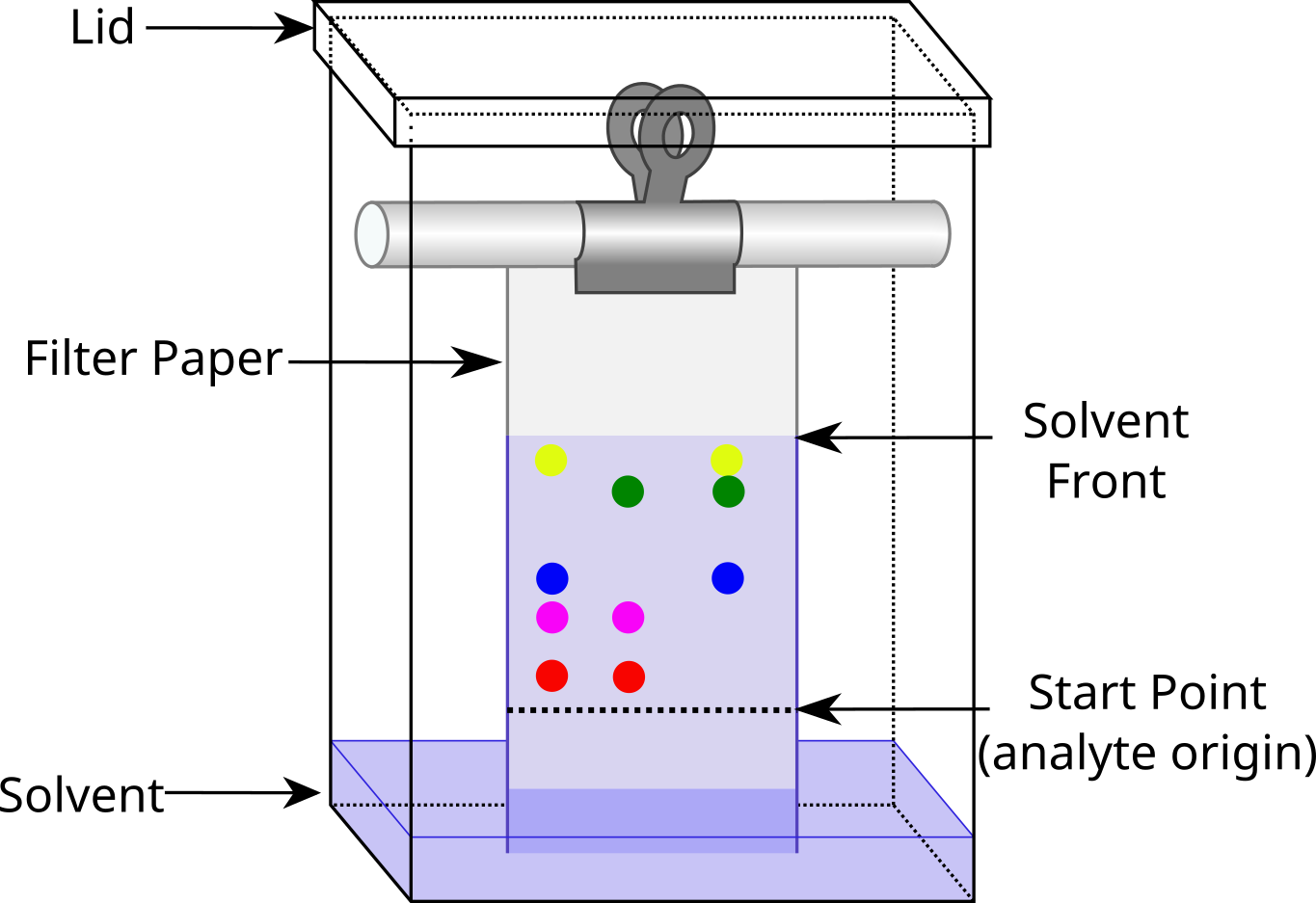
The distance that the analyte migrates along the paper related to the total distance that the solvent or mobile phase moves is called the Retention Factor or RF.
Tags: quantitative reasoning, guided inquiry


