Contents [hide]
Introduction: Carbohydrates
Carbohydrates serve 2 major functions: energy and structure. As energy, they can be simple for fast utilization or complex for storage. Simple sugars are monomers called monosaccharides. These are readily taken into cells and used immediately for energy. The most important monosaccharide is glucose (C6H12O6), since it is the preferred energy source for cells. The conversion of this chemical into cellular energy can be described by the equation below:
C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + energy
Long polymers of carbohydrates are called polysaccharides and are not readily taken into cells for use as energy. These are used often for energy storage. Examples of energy storage molecules are: amylose or starch (plants) and glycogen (animals). Some polysaccharides are so long and complex that they are used for structure like cellulose in the cell walls of plants. Cellulose is very large and practically indigestible, making it unsuitable as a readily available energy source for cells.

Many monosaccharides such as glucose and fructose are reducing sugars, meaning that they possess free aldehyde or ketone groups that reduce weak oxidizing agents such as the copper in Benedict’s reagent. The double bond in the carbonyl group is a source of electrons that can be donated to something else. That is to say, those electrons can be “lost” by the sugar and “gained” by another chemical. Benedict’s reagent contains cupric (copper) ion complexed with citrate in alkaline solution. Benedict’s test identifies reducing sugars based on their ability to reduce the cupric (Cu2+) ions to cuprous oxide (Cu+) at basic (high) pH. Cuprous oxide is green to reddish orange. Roughly speaking, reduction is a type of chemical reaction that is paired with oxidation. In oxidation/reduction reactions (RedOx), some chemical loses electrons (oxidized) to another chemical that gains them (reduced). We remember whether a compound is reduced or gained by using the pnemonic: LEO goes GER or Loss of Electrons is Oxidation & Gain of Electrons is Reduction.

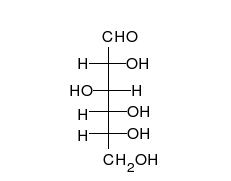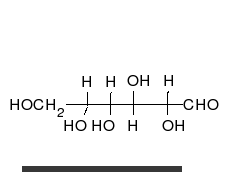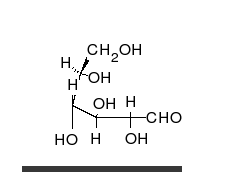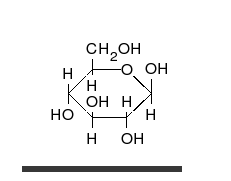
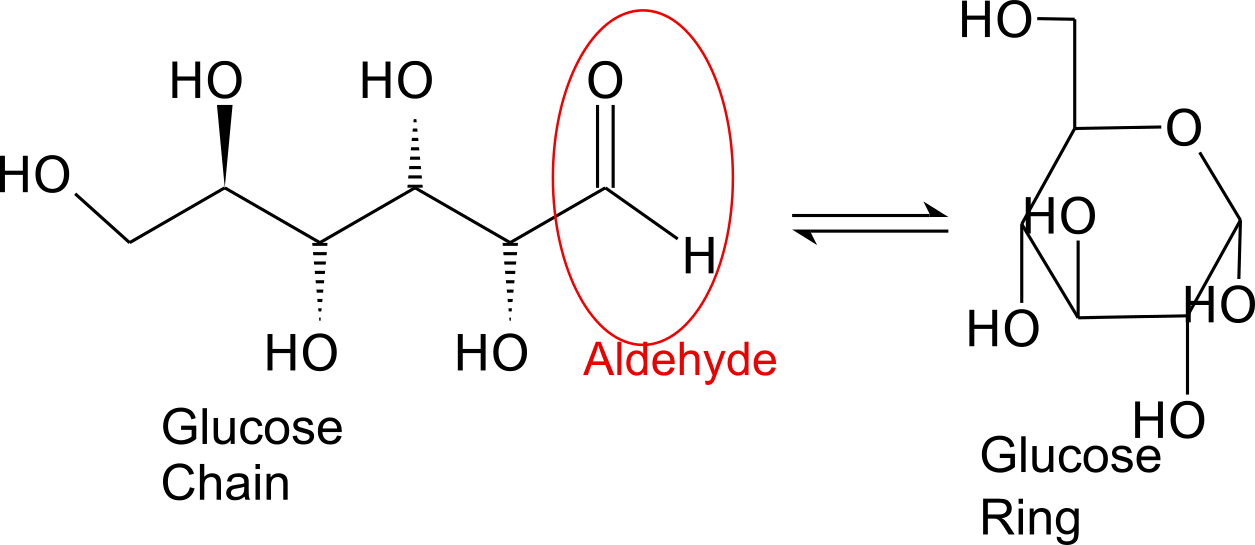 Monosaccharides are capable of isomerizing. This means they alternate in structure from a linear chain to a ring form in solution. In the chain form, the aldehyde is free to donate (lose) electrons to reduce another compound. When monosaccharides undergo dehydration synthesis to form polymers, they can no longer isomerize into chains with free aldehydes and are unable to act as reducing sugars. Green color indicates a small amount of reducing sugars, and reddish orange color indicates an abundance of reducing sugars. Non-reducing sugars produce no change in color (i.e., the solution remains blue).
Monosaccharides are capable of isomerizing. This means they alternate in structure from a linear chain to a ring form in solution. In the chain form, the aldehyde is free to donate (lose) electrons to reduce another compound. When monosaccharides undergo dehydration synthesis to form polymers, they can no longer isomerize into chains with free aldehydes and are unable to act as reducing sugars. Green color indicates a small amount of reducing sugars, and reddish orange color indicates an abundance of reducing sugars. Non-reducing sugars produce no change in color (i.e., the solution remains blue).
Note: Cu2+ has fewer electrons than Cu+.
When monosaccharides undergo dehydration synthesis to form polymers, they can no longer isomerize into chains with free aldehydes and are unable to act as reducing sugars. Green color indicates a small amount of reducing sugars, and reddish orange color indicates an abundance of reducing sugars. Non-reducing sugars produce no change in color (i.e., the solution remains blue).
Structural Carbohydrates
In food, more complex carbohydrates are derived from larger polysaccharides. These larger carbohydrates are fairly insoluble in water. Dietary fiber is name given to indigestible materials in food most often derived from the complex carbohydrates from vegetable material. Some of this material serves the plants as a structural component of the cells and is completely insoluble. Cellulose is the major structural carbohydrate found in plant cell walls. Similarly, animals and fungi have structural carbohydrates that are composed of the indigestible compound called chitin. We will not be testing for these items.