Contents
How Do Atoms Bond?
Understanding Chemistry in 3D
- Click on the image above to launch the simulation
- Choose “Real Molecules”
- Click on “Show Bond Angles”
- you may also de-select “Show Lone Pairs”
- Drag the atoms to understand the 3-D shape
- Choose CH4 from the drop-down menu of molecules
- CH4 is methane, the simplest organic molecule
- notice the restraints on the physical space occupied by the atoms as you drag the atoms around
- remember these bond angles and constraints when we explore organic chemistry
- toggling between “Model” and “Real” will illustrate if the position of the atoms are influenced by other factors we don’t see
- Choose NH4 from the drop-down menu
- NH4 is ammonia, a polar solvent
- NH4 is NOT an organic molecule
- Toggle the lone pairs to see how these electrons play a role in the positioning of atoms
- Choose CO2 from the drop-down
- explore this non-organic molecule to visualize the effects of double bonds on geometry
Covalent Bonding in Methane, Water and Ammonia
Organic Chemistry
Living things are composed of organic molecules primarily made up of the elements carbon and hydrogen. Molecules of hydrogen and carbon (referred to as hydrocarbons) have the property of being non–polar. Yet 70- 90% of cells are composed of water (a polar compound). Polar substances mix with other polar substances. Likewise, non-polar substances interact with other non-polar compounds. Polar and non-polar compounds are immiscible (unable to mix).
So how do cells keep from falling apart in a water environment?
Functional groups are clusters of atoms in a group that impart a new “function” to the compound they are attached to. Hydrocarbons in cells have functional groups attached to them that permit them to interact with the water environment of the cell. These functional groups also define the type of molecule it is based on the characteristics of those groups.
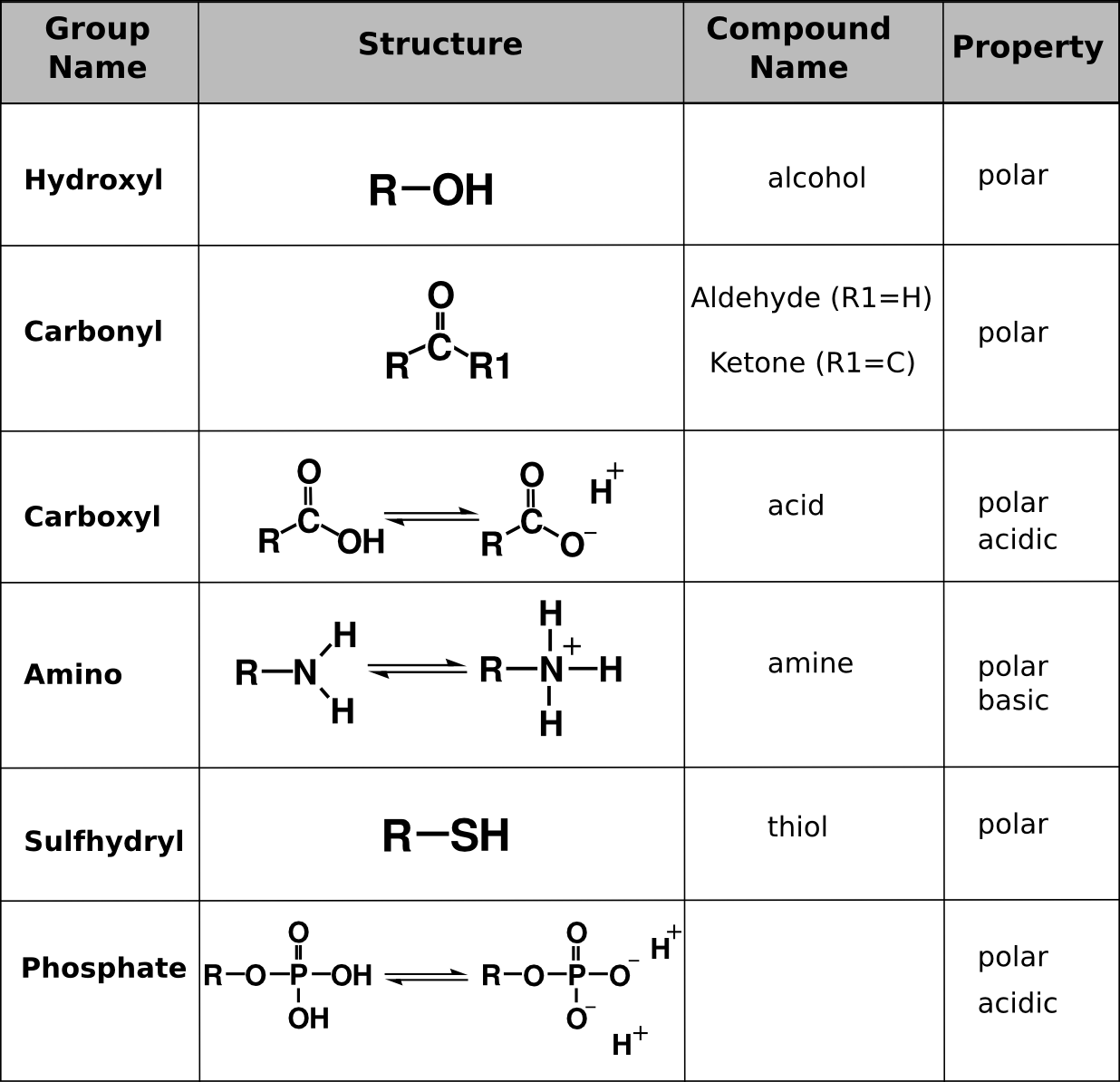
How are macromolecules assembled?
The common organic compounds of living organisms are carbohydrates, proteins, lipids, and nucleic acids. Each of these are macromolecules or polymers made of smaller subunits called monomers. The bonds between these subunits are formed by a process called dehydration synthesis. This process requires energy; a molecule of water is removed (dehydration) and a covalent bond is formed between the subunits. Because a new water molecule is formed, this is also referred to as condensation. The opposite where water and energy are used to break apart polymers into simpler monomers is called hydrolysis (hydro– water, lysis– to break or split).

Where do we find macromolecules?

Additional Resources
Vocabulary
|
|