Contents [hide]
Reading
- 6.1 Energy and Metabolism (OpenStax CNX)
- 6.2 Potential, Kinetic and Free Energy (OpenStax CNX)
- 6.3 Thermodynamics (OpenStax CNX)
- 6.4 ATP (OpenStax CNX)
- 6.5 Enzymes and Catalysis (OpenStax CNX)
Learning Objectives
- Contrast potential and kinetic energy, and identify different forms in which energy can exist.
- Apply the first and second laws of thermodynamics to living organisms and to the ecosphere.
- Describe the energy dynamics of a reaction that is in equilibrium; explain the meaning of chemical formulas and equations and what they convey about reactants and products.
- Distinguish between endergonic and exergonic reactions, and explain how they may be coupled so that the second law of thermodynamics is not violated.
- Relate the chemical structure of ATP to its role in cellular metabolism.
- Explain the relationship between enzymes, catalysts, and the energy of activation.
- Use the terms active site, substrate, and induced-fit to explain how an enzyme converts a substrate to a product.
- Define the term enzyme cofactor, and list the two main types of cofactors found in cells.
- Explain what is meant by a biochemical pathway, and state three reasons why such pathways are advantageous for cells.
- List the three components of an ATP molecule, and show how ATP, ADP, and AMP can be inter-changed.
- Use the terms hydrolysis, coupled reactions, and photophosphorylation to explain why ATP is called the cell’s energy currency.
- Write a general equation illustrating hydrogen and electron transfer from a substrate to a hydrogen acceptor such as NAD+ or FAD+.
Slide Show
Energy Cycling
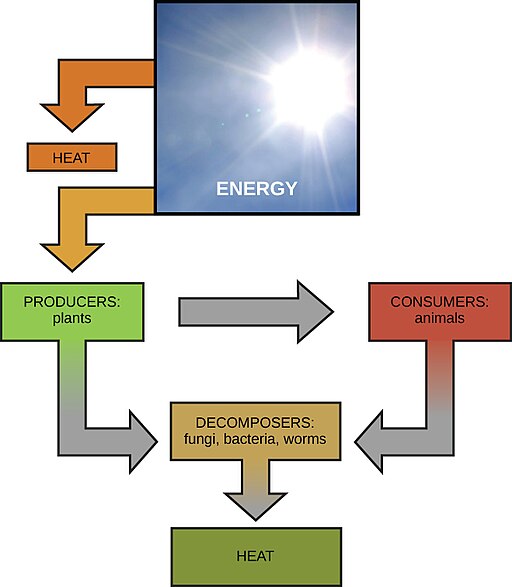
Solar energy powers life on Earth. Credit: OpenStax CNX [CC-BY 4.0]

Autotrophs produce chemical energy that yield organic compounds that sustain heterotrophs. Credit:Mikael Häggström, Laghi l, BorgQueen, Benjah-bmm27, Rkitko, Bobisbob, Jacek FH, Laghi L and Jynto [CC-BY-SA 3.0]
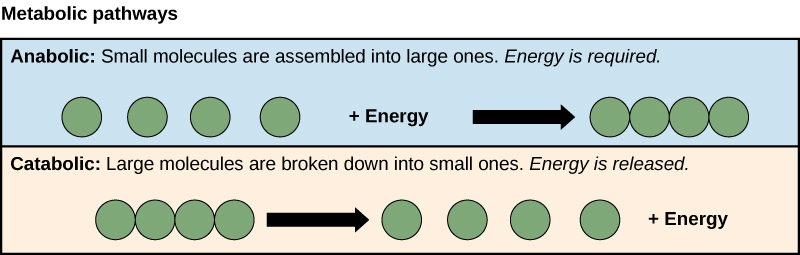
Metabolism consists of the sum total of chemical reactions within cells. They consist of Anabolic (building or synthetic) and Catabolic (degrading) pathways. Credit: OpenStax CNX [CC-BY 4.0]
Energy and catalysts

Exergonic reactions release energy while Endergonic reactions take energy in. Credit: OpenStax CNX [CC-BY 4.0]
In Biological systems, energy is roughly defined as the capacity to do work. Molecules are held together by electrons. Breaking and building these bonds requires an input of energy. The energy needed to initiate such reactions is referred to as activation energy (EA). Sometimes the necessary energy to initiate a reaction is so great, that it greatly limits the likelihood of the reaction ever occurring. Catalysts are chemicals that take part in facilitating reactions by reducing the energy of activation. If the activation energy is reduced, the likelihood of a reaction occurring is greatly enhanced. In cells, the catalysts are often made of proteins and called enzymes.
![By Originally uploaded by Jerry Crimson Mann, vectorized by Tutmosis, corrected by Fvasconcellos (en:Image:Activation2.png) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons reaction coordinates](https://openlab.citytech.cuny.edu/bio-oer/files/2015/09/Activation2_updated.svg_.png)
Reaction coordinate of an exothermic reaction with and without an enzyme. The enzyme reduced the EA to facilitate the likelihood that the reaction occurs. This catabolic reaction breaks complex things down, thus increasing entropy and releasing energy into the system.
Enzymes
Reactants in enzymatic reactions are called substrates. They have an imperfect fit to a binding domain of the enzyme called the active site. Substrate binding to this active site induces a change in the shape of the protein that coordinates the substrate into a transition state that will reduce the amount of EA required for the reaction to go to completion. The induced fit of the protein also aids in coordinating other cofactors or coenzymes that will aid in the reaction.

Induced fit model of enzymes and substrates. The active site of the protein is an imperfect match for the substrate. Intermolecular interactions between the enzyme and substrate induce a new fit that facilitates the formation of a transition state and results in the catalysis of the reaction.
The reaction follows the standard flow where the Enzyme (E) and the Substrate (S) interact to form an Enzyme-Substrate Complex (ES). The ES then dissociates into Enzyme and the resultant Product (P)
E + S ⇒ ES ⇒ E + P
The induced fit of the enzyme-substrate complex coordinates the transition state to facilitate the reaction. This induced fit occurs through non-covalent means that result in a tugging on the molecules (an application of energy) while molecules are coaxed into the reactions.

Hexokinase enzyme interacts with an ATP and a hexose. These interactions alter slightly the structure of the enzyme (induced fit). This pulling on the enzyme and the substrates aids in catalyzing the reaction through coordinating the molecules, sometimes with the aid of cofactors and coenzymes. The yellow sphere represents the cofactor Mg2+