Contents [hide]
Reading
- Loading...
- 2.2 Water (OpenStax CNX)
Learning Objectives
- List the significance of water in living systems – indicating at least three important features.
- Differentiate between electrolytes and non electrolytes and construct a chart of common examples of both – utilizing a list of common chemicals to be supplied to him/her.
- Compare the degree of ionization between solutions of: NaCl, HCl, tap water, sucrose and starch.
- Given a list of salts, identify salts and possible buffers – illustrating their role in the living cell.
- Construct a pH scale, given a series of different concentrations of solutions, indicate:
- the scale or range of both the hydrogen and hydroxyl ion concentrations
- the location of weak and strong bases; the location of weak and strong acids.
- the pH range as seen in the human body.
Polar Covalent Bonds
H2O is a polar covalent molecule. The Bonds between the H atoms and the O atom arise from sharing electrons. These shared electrons form to satisfy the octet rule. However, Oxygen is a “selfish” sharer. This electronegative aspect of Oxygen means that the electrons of the H2O molecule preferentially associate near the Oxygen atom, creating partial charges. We indicate this by placing a δ– near the O and δ+‘s near the H atoms. These partial charges make the H2O polar.

The electron cloud around a water molecule lingers around the oxygen molecule to render it partially negative. Red illustrates the partial negative end of the molecule while blue indicates the partial positive.
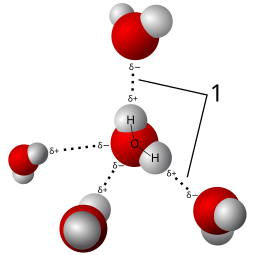
Credit: Qwerter, sevela.p, snek01 [CC-BY-SA 3.0]
Because of this polarity, H2O molecules arrange in a highly structured way. Use the following simulation to explore polarity of molecules.
These weak associations that arise from the polar:polar attractions are referred to as Hydrogen Bonds (H-bonds). While independently weak, the summation of all the H-bonds are very strong. These associations give rise to the special properties of water: surface tension, cohesion, adhesion, high specific heat capacity.
Polar materials mix with polar materials. Things that can dissolve in water are also polar and referred to as being hydrophilic (hydro– water, philic– liking). Non-polar substances do not interact or mix with polar solvents and are referred to as hydrophobic (hydro– water, phobic– hating). Since carbon and hydrogen share electrons equally, organic compounds are non-polar. Oil is a hydrocarbon that does not mix well with water or vinegar. Vinegar, however is a polar compound that interacts with water. Detergents are called amphiphilic (amphi– both; philic– liking) because they have portions that are non-polar and portions that are very polar. Detergents can therefore dissolve in hydrocarbons and water. Water alone cannot effectively remove oil from your skin, but a detergent can dissolve the oil and carry it away in water.
Surface Tension
Surface tension presents as an invisible film that encompasses the surface of water. The attractive forces arising from the intermolecular cohesion holds the surface of water together.

This paperclip would sink if it broke through the surface of the water. Credit: Alvesgaspar [CC-BY-SA 3.0]

This water strider is not on top of the water because it is light. It has not broken through the surface of the water and is therefore, on top of the water. Credit: Markus Gayda [CC-BY-SA 3.0]

Clayton Anderson demonstrates the cohesive nature of water in microgravity. Water clings to itself through hydrogen bonding. [CC0]
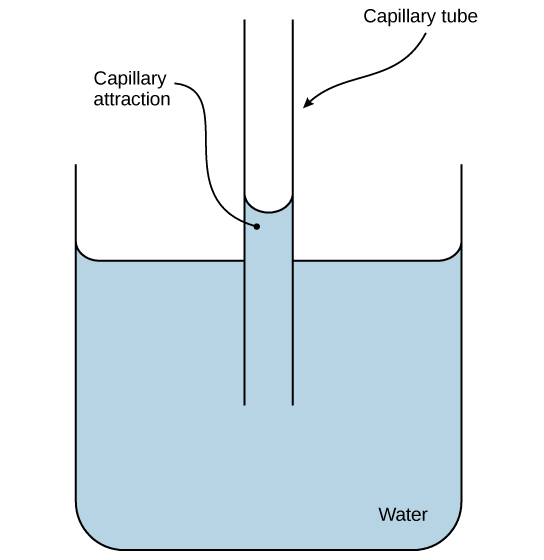
Hydrogen bonds of water can drive adhesion as evidenced by capillary action. Credit: CNX OpenStax [CC BY 4.0]