Contents [hide]
Learning Objectives
- Name the four major types of organic molecules found in living organisms and tell what they all have in common.
- Define the term carbohydrate and distinguish between a monosaccharide, a disaccharide, and a polysaccharide.
- Describe the process of dehydration synthesis and hydrolysis, and explain why they are important in living organisms.
- Define the term lipid and list some of the roles played by lipids in the cell.
- Explain the difference between (a) a saturated and an unsaturated fatty acid, (b) a fat an an oil, (c) a phospholipid and a glycolipid, and (d) a steroid and a wax.
- Draw the basic structure of an amino acid, and explain the relationships between amino acids, proteins, and peptide bonds.
- Describe the levels of organization of protein in terms of its primary, secondary, tertiary, and quaternary structures.
- Define the term nucleic acid, give the subunits of a nucleotide, and name the two main types of nucleic acids found in living organisms.
- Explain why ATP is important and describe its general structure.
Understanding Chemistry in 3D
Organic Chemistry
Living things are composed of organic molecules primarily made up of the elements carbon and hydrogen. Molecules of hydrogen and carbon (referred to as hydrocarbons) have the property of being non–polar. Yet 70- 90% of cells are composed of water (a polar compound). Polar substances mix with other polar substances. Likewise, non-polar substances interact with other non-polar compounds. Polar and non-polar compounds are immiscible (unable to mix).
So how do cells keep from falling apart in a water environment?
Functional groups are clusters of atoms in a group that impart a new “function” to the compound they are attached to. Hydrocarbons in cells have functional groups attached to them that permit them to interact with the water environment of the cell. These functional groups also define the type of molecule it is based on the characteristics of those groups.
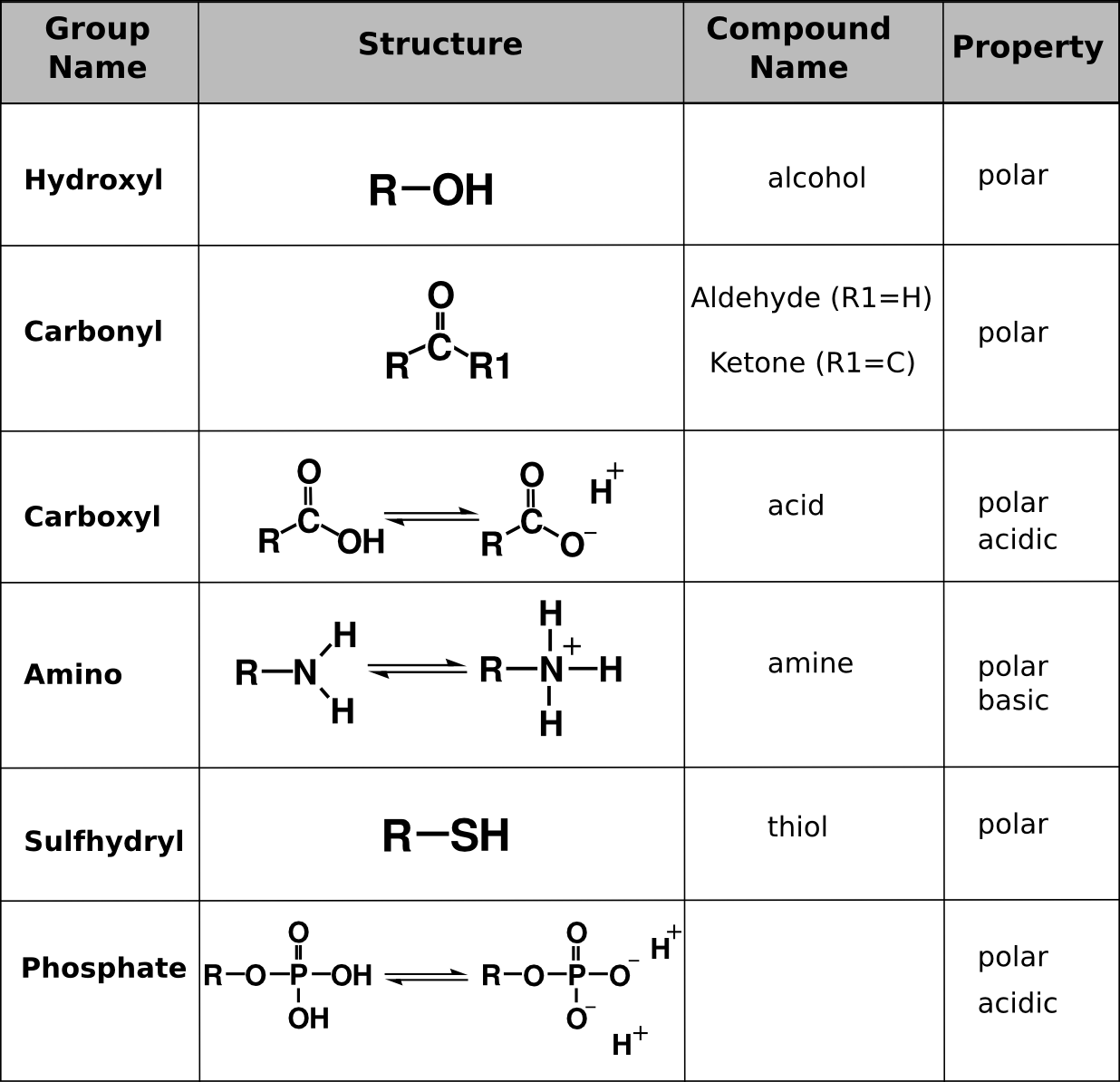
Functional Groups. Functional groups commonly found in organic compounds. R is a placeholder chemical that can be anything (like a hydrocarbon chain). The functional groups in this table are those that add polar or charged properties to the hydrocarbon chains. Bi-directional arrows indicate that those functional groups dynamically ionize and reach an equilibrium in solution.
How are macromolecules assembled?
The common organic compounds of living organisms are carbohydrates, proteins, lipids, and nucleic acids. Each of these are macromolecules or polymers made of smaller subunits called monomers. The bonds between these subunits are formed by a process called dehydration synthesis. This process requires energy; a molecule of water is removed (dehydration) and a covalent bond is formed between the subunits. Because a new water molecule is formed, this is also referred to as condensation. The opposite where water and energy are used to break apart polymers into simpler monomers is called hydrolysis (hydro– water, lysis– to break or split).