Contents
Learning Objectives
- Differentiate between an atom, an element, a compound, a molecule and subatomic particles; given a list of such terms, he/she should be able to match them with appropriate phrases or synonyms – selecting at least 10 of a choice of 15 possibilities.
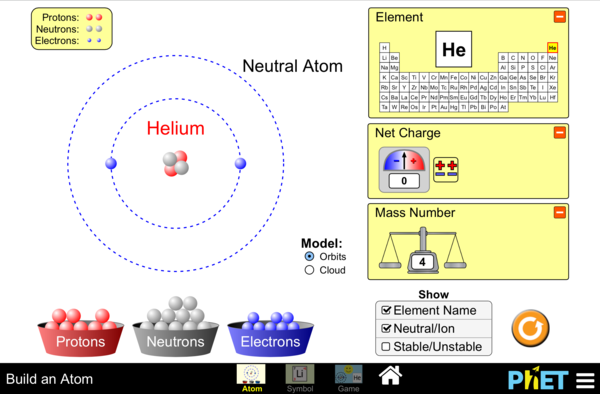
- Determine the pertinent characteristics (atomic number, atomic mass, location of protons, neutrons, electrons) of 15 of the first 20 elements in the periodic table.
- Indicate the relative chemical activities of eight out of ten of a given list of atoms (such as hydrogen, helium, boron, carbon, nitrogen, oxygen, sodium, chlorine, potassium, and calcium).
- Given at least three of five possible features of a theoretical atom – state the atomic mass and number.
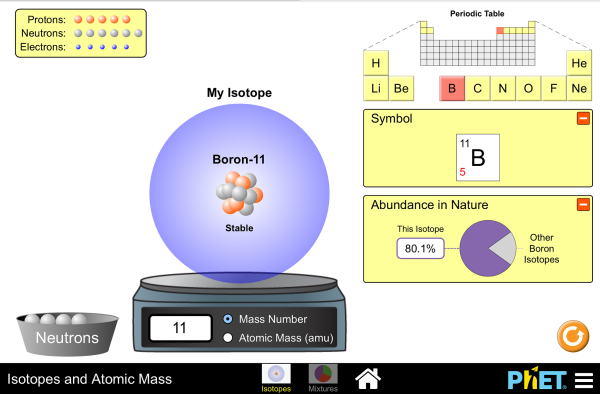
- Indicate the chemical and biological significance of isotopes, selecting two from a list of five supplied to him/her.
- Define and give examples of electrovalence, covalence, non polar, and polar covalent bonds, ionic bonding and hydrogen bonding.
- Contrast between and give four (4) examples of inorganic and organic compounds of biological significance.
Atoms and Molecules
Build atoms using subatomic particles
Understand Atomic Mass and Isotopes
The Bohr Model and Electron Configurations
How do Bonds Form?
Covalent Bonding
Isomers