Life as we know it
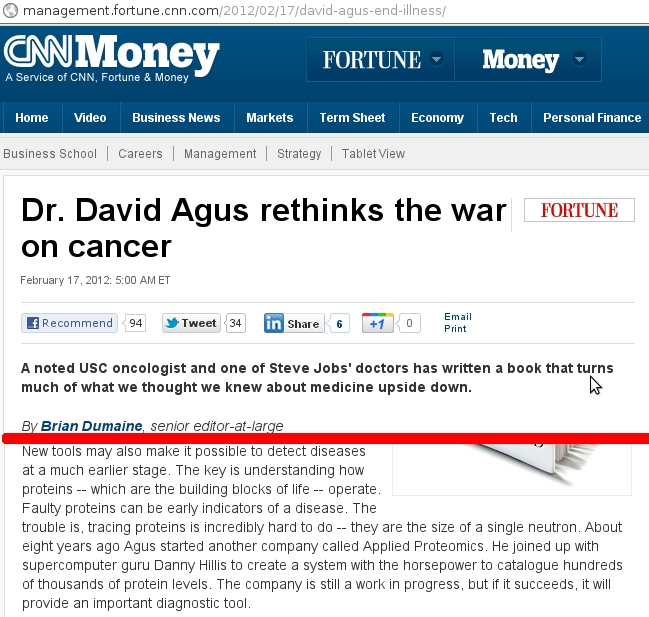
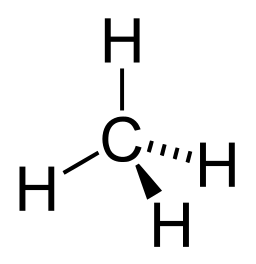
We have briefly studied organic chemistry to understand the basis of Carbon-based life on our planet. Because Carbon has a valence of 4, we can understand the scientific rationalization of imagining Silicon-based life-forms in Science Fiction storylines. For an expanded view and understanding of Silicon versus Carbon life, please read this article at Treknews.

The study of life outside of Earth was referred to as exobiology. Now, the field of Astrobiology has emerged to consolidate fields of astronomy, astrogeology, molecular biology, ecology and chemistry in order to understand life within the universe. While Carbon is the basis of our Biology, we would imagine that life could arise in other forms given a different environment than ours. Life forms, as we know them, are predominantly composed of Hydrogen, Carbon, Oxygen, Nitrogen, Phosphorus and Sulfur. Given our brief introduction to chemistry towards the goal of understanding basic mechanisms of life, we can begin to understand other hypothetical biochemistries.
Not so hypothetical?
In 2010, Felisa Lauren Wolfe-Simon of the NASA Astrobiology Institute published a paper regarding and extremophile bacteria called GFAJ-1 from Mono Lake. Mono Lake is hypersaline (very salty) and basic in pH. The extreme conditions of the lake provided a unique environment on our planet that has given rise to specialized organisms. The paper published in the journal Science indicated that these bacteria could utilize Arsenic if starved of Phosphorus.
What’s the significance of this finding? Well, Arsenic is typically poisonous to life as we know it. Why would that be so? If we examine the location of Arsenic and Phosphorus on the periodic table, we see that they are in Group V. This means that they have a valence of 5. With 5 free electrons in the outer shell, we would suspect a similarity of reactivity and structure formation as we’ve already indicated between Carbon and Silicon. In our biology, we see that much of the Phosphorus in our body is a component of Phosphate, which is necessary for building our genetic material and for cellular energetics.


As we can see from the structures of Arsenate and Phosphate, they are similar. Because of this, Arsenate is highly toxic. Our cellular machinery recognizes it as something like Phosphate, but cannot utilize it the same way. If this newly identified bacteria could in fact utilize and incorporate Arsenate, it would represent a demonstrated hypothetical biochemistry that defied what was already known.
Nullify your hypothesis
In the scientific method, we fortify and rigorously test our hypotheses by attempting to nullify them in multiple ways. This methodology removes experimental biases from our interpretations. In the case of GFAJ-1, the outcome was so surprising and contrary to conventional biology that it attracted many scientists to carefully scrutinize the data that gave rise to the conclusion of Arsenate incorporation. At this time, criticism and analysis of the data have given rise to alternative interpretations and a second group has repeated the experiments without observing any Arsenic incorporation.
Your life
Some of you may be wondering how this plays any significant role in your life. Take a look at this topic from one of our nutrition classes at Citytech.