For Dr. Joasil’s Anatomy and Physiology class. I substituted for the lectures on the endocrine system. You can download the slides here
Sugar Follow-up
Thanks to all who have taken part in Assignment 2. Last Sunday, 60 Minutes on CBS aired a new piece on Robert Lustig’s war on sugar with additional experts who talk about the problems with excessive sugar in the diet. Check it out at the CBS site. Remember, carbohydrates are necessary for cells to function. The message of moderation and reduction in added sugars to processed foods does not equate to complete starvation. The segment focuses on multiple diseases:
- Diabetes
- Obesity
- Heart Disease
- Hypertension
- Cancer
Keep in mind the obviousness of obesity and diabetes in the context of excessive carbohydrate consumption. But also look at the effects of simple carbohydrates in contributing to cancer, hypertension and heart disease.
Dr. Gupta is interviewed in a short segment about how people need to learn to be more concious about their consumption habits. What are your thoughts?
Carbs are evil?
Dr. Robert Lustig of UCSF has been on a mission to advocate for the reduction of processed food consumption. He particularly targets simple carbohydrates — specifically the sugar fructose. Fructose is a pentose sugar that is found abundantly in nature. In fact, it’s the major form of sugar found in fruits. We all know that fruits are good for you, right? It’s true! But Dr. Lustig argues that the consumption of fruit is beneficial due to the more complex and less digestable carbohydrates that come in the form of dietary fiber. Well what’s the difference? Absorption of nutrients during digestion is regulated by the environment of the food. Fiber regulates the rate of simple carbohydrate absorption and entry into the bloodstream. So why specifically fructose? The common sugar that is utilized by cells is the hexose sugar glucose. Why doesn’t he think that is bad? He does think it is when not moderated. Just as we don’t simply consume pure glucose even though that is what our cells utilize, he argues that too much sugars –glucose, fructose and the disaccharide sucrose– is the major source of disease risk in this country. It all stems from obesity.
Recently on NPR Science Friday, Dr. Lustig spoke about his advocacy for a “Soda tax” to recoup revenue used to combat illnesses brought on by obesity (Download the Podcast here). The logic is that obesity can be combated through inducing moderation fo sugar intake. He likens it to taxes imposed on cigarettes and nicotine products and assures the public that people will learn to accept increased prices for these products. So why is there an increase of obesity in this country? Food prices in the United States are relatively low. Additionally, products that are made from corn (as well as other crops) are highly subsidized by the federal government in an effort to reduce food costs. The side-effect is the abundance of corn syrup found in many food items that are high in fructose.
Carbohydrates versus Lipids
Sugars are carbohydrates. Plants typically store simple sugars in polymers for temporary storage of energy in the form of starch. Animals will store energy in sugar form as the polymer known as glycogen in the liver and muscle. What does any of this have to do with fat? Fat is a type of lipid composed of a glycerol molecule and 3 fatty acid chains. We refer to them as triglycerides. As we can see, carbohydrates and lipids are quite different. Fats are used to store energy for the long-term. When there is an abundance of energy that is not immediately needed, it is converted into long-term storage as fat. Sugar does not equal fat! An abundance of it will eventually lead to fat deposition if that energy is not utilized immediately.
The opposing view
Those who oppose Dr. Lustig often cite the fact that sugar is sugar, no matter what the source. Corn syrup is still just sugar. The corn lobby will constantly remind you of this in televised commercials. Here is a summary of an opposing view at Science Daily. The study focuses on the caloric value of fructose. This isn’t exactly what Dr. Lustig argues about. He merely suggests that the abundance of sugar is so great, that it is too easily consumed. He often states that sugar is a luxury (of yesteryear) that should be moderated. How does the Science Daily article change your views on the argument?
Food for thought
Think about the questions below. If you do the assignment, please post it in the appropriate place in the comments for the assignment.
- Weigh in on your opinion in this debate and refer to specific arguments from the podcast and the Science Daily article
- Do you feel that having excessive amounts of sugary products is deleterious towards your well-being?
- Do you think taxation will make a difference in the habits of consumers that will effectively reduce obesity?
- What are the alternatives to taxation that would/could be implemented instead of a “soda tax”?
- What are your opinions regarding food subsidies and the abundance of low cost food of questionable “quality”?
- Compare a high quality and balanced meal versus a “McMeal”
New role for Spider Silk

Spider silk is a protein fiber spun from spiders to make webs. Silk is renowned for it’s high tensile strength that is equivalent to that of steel (weight for weight). On a macroscopic level, silk is often found as gossamer fibers. A closer look at the protein structure reveals a repetitive block structure consisting of β-sheets.

The primary amino acid sequence contains blocks of Alanine and Glycine. the antiparallel strands assemble so the -C=O from one amino acid residue hydrogen bonds with the -N-H of the opposite strand. Individually, H-bonds are weak, but assembled in bulk, they provide a strength and flexibility that has been utilized in fine textiles for centuries.
A new article has been published in the journal Physical Review Letters describing a new method of obtaining large quantities of spider silk to generate musical cordage. Traditionally, violin strings were made from fibers obtained from animal (cattle) intestines and known as catgut. Newer instruments often replace catgut strings with steel, nylon and polymer strings. With this came differences in the quality of sound but enhanced resilience. Spider silk strings also provide a slightly different sound quality with reduced resilience compared to synthetic cordage but outlast catgut. Some professional violinists have been quoted as enjoying the sound quality of the spider silk strings.
Astrobiology: Weird chemistry… weirder biology
Life as we know it
We have briefly studied organic chemistry to understand the basis of Carbon-based life on our planet. Because Carbon has a valence of 4, we can understand the scientific rationalization of imagining Silicon-based life-forms in Science Fiction storylines. For an expanded view and understanding of Silicon versus Carbon life, please read this article at Treknews.
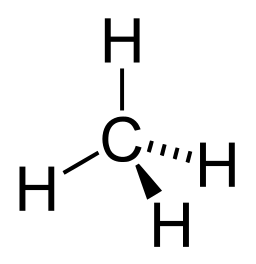

The study of life outside of Earth was referred to as exobiology. Now, the field of Astrobiology has emerged to consolidate fields of astronomy, astrogeology, molecular biology, ecology and chemistry in order to understand life within the universe. While Carbon is the basis of our Biology, we would imagine that life could arise in other forms given a different environment than ours. Life forms, as we know them, are predominantly composed of Hydrogen, Carbon, Oxygen, Nitrogen, Phosphorus and Sulfur. Given our brief introduction to chemistry towards the goal of understanding basic mechanisms of life, we can begin to understand other hypothetical biochemistries.
Not so hypothetical?
In 2010, Felisa Lauren Wolfe-Simon of the NASA Astrobiology Institute published a paper regarding and extremophile bacteria called GFAJ-1 from Mono Lake. Mono Lake is hypersaline (very salty) and basic in pH. The extreme conditions of the lake provided a unique environment on our planet that has given rise to specialized organisms. The paper published in the journal Science indicated that these bacteria could utilize Arsenic if starved of Phosphorus.
What’s the significance of this finding? Well, Arsenic is typically poisonous to life as we know it. Why would that be so? If we examine the location of Arsenic and Phosphorus on the periodic table, we see that they are in Group V. This means that they have a valence of 5. With 5 free electrons in the outer shell, we would suspect a similarity of reactivity and structure formation as we’ve already indicated between Carbon and Silicon. In our biology, we see that much of the Phosphorus in our body is a component of Phosphate, which is necessary for building our genetic material and for cellular energetics.


As we can see from the structures of Arsenate and Phosphate, they are similar. Because of this, Arsenate is highly toxic. Our cellular machinery recognizes it as something like Phosphate, but cannot utilize it the same way. If this newly identified bacteria could in fact utilize and incorporate Arsenate, it would represent a demonstrated hypothetical biochemistry that defied what was already known.
Nullify your hypothesis
In the scientific method, we fortify and rigorously test our hypotheses by attempting to nullify them in multiple ways. This methodology removes experimental biases from our interpretations. In the case of GFAJ-1, the outcome was so surprising and contrary to conventional biology that it attracted many scientists to carefully scrutinize the data that gave rise to the conclusion of Arsenate incorporation. At this time, criticism and analysis of the data have given rise to alternative interpretations and a second group has repeated the experiments without observing any Arsenic incorporation.
Your life
Some of you may be wondering how this plays any significant role in your life. Take a look at this topic from one of our nutrition classes at Citytech.
Science Literacy: Chemistry
On February 17th, CNN published an online article about cancer treatment and argued towards prophylactically attacking cancer before the state or process of cancer arises. It originally contained an error.
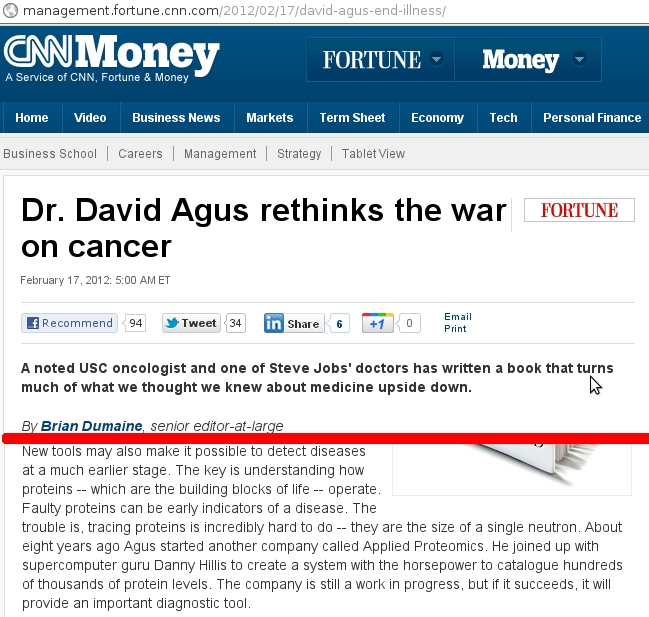
Article containing an error from CNN. Red line indicates a cut in the text to focus on the incorrect passage. (Screencapture for educational and scholarly discussion, thus qualifies as Fair Use)
A neutron is a sub-atomic particle. Sub-atomic indicates that it is smaller than an atom. We know that atoms build molecules and proteins are biologically important macromolecular polymers. We know that a proton is a sub-atomic particle that has an approximate mass of a neutron. From the context of the article, this is not where the error lies –proton in place of protein.
Some time after the article was published online, there was an edit to rectify the error:
New tools may also make it possible to detect diseases at a much earlier stage. The key is understanding how proteins — which are the building blocks of life — operate. Faulty proteins can be early indicators of a disease. The trouble is, tracing proteins is incredibly hard to do — they have to be examined at the level of a single neutron. About eight years ago Agus started another company called Applied Proteomics. He joined up with supercomputer guru Danny Hillis to create a system with the horsepower to catalogue hundreds of thousands of protein levels. The company is still a work in progress, but if it succeeds, it will provide an important diagnostic tool.
The passage now reads that proteins must be studied at the level of a single neutron. If a protein is a macromolecule and a neutron is a sub-atomic particle:
- What could that possibly mean to trace proteins at the level of the neutrons?
The passage then states that Applied Proteomics is cataloging hundreds of thousands of protein levels (in magenta). As a lay scientist:
- What are your interpretations of this last statement?
- How would you interpret the work of Applied Proteomics from this article?
- How would you interpret the work of Applied Proteomics from from their website?
As a scientist, I would remark that this statement is gibberish, especially in the context of the rest of the article. Often times, we come across improperly written statements. We understand that science is very technical, but understanding some basic concepts, we can identify the faults in improper statements. We can further attempt to clarify improper statements by seeking out additional information. Here, since the statements are so befuddling, we can then turn to the primary source of information –Applied Proteomics.
Paper Chromatography
In the Biology Lab, we separated the dyes that are present in chocolate candies. We utilized the solubility in a solvent versus the retention on a stationary phase using paper chromatography. Please calculate the Rf:
| Color | Distance (cm) | Rf | |
| Red | Red 1.5 | ||
| Green | Blue 7.5 Yellow 4.3 |
||
| Orange | Red 1.4 Yellow 4.5 |
||
| Yellow | Yellow 4.6 | ||
| M&M Orange | Red 1.5 Yellow 4.6 |
||
| M&M Red | Red 1.3 | ||
| M&M Brown | Red 1.7 Yellow 3.1 Blue 7.1 |
||
| M&M Green | Yellow 1.7 Blue 6.7 |
||
| Solvent Front | Front 8.6 | ||
The mobile phase was a saltwater solution. What do we learn from the Rf of each food coloring dye assuming they are of equivalent mass and/or size?
pH
Acids and Bases
We can call any compound that adds H+ ions (a free proton) into solution an acid. Along with this, we would expect that any compound that would decrease the concentration of free H+ of a solution as a base. pH is the power of H+ of a solution. We define this power as a molar concentration of H+ in solution. This concentration invariably ends up being a relatively small number (though great in absolute numbers) and is expressed as a decimal number. Because the range of the concentrations is so great, we express these numbers as logarithmic numbers to avoid writing many 0’s after the decimal and to facilitate communicating the concentration. Since these numbers are so (relatively) small, we use the negative logarithm to describe this concentration.
Mathematically defined, pH = -Log10[H+]
The pH scale ranges so that anything below pH 7 is acidic and anything above pH 7 is alkaline. So a smaller number is more acidic. But didn’t we just state that something acidic contains more H+ ions? Remember, because we are dealing with a negative Logarithm, this means the concentration is higher.
Now you’re just talking crazy!
If we have a quantity that is 102, we know that translates into 100. Just as if we have a quantity of 104, we know that translates into 10000. Just as it becomes inconvenient to keep writing all those 0’s, it’s really impractical to write many many 0’s after a decimal. It’s really hard to talk about too! So we likewise will express numbers like 0.0001 as 10-4. A logarithm is the reverse function of an exponent. Therefore:
- Log10(0.0001) = -4
- Log1010-4 = -4
So how do we define a solution that is pH 2? Well, we already decided that this solution is below pH 7 — making it an acid. But what does this mean in terms of H+ ion concentration?
Let’s work this out algebraically:
- pH = -Log10[H+] Let’s bring the (-) over to the other side
- -(pH) = Log10[H+] Now let’s reverse the Log → base 10
- 10-(pH) = [H+] Plug in the pH → molar concentration of [H+]
As we can now see, a solution of pH 2 is acidic because the molar concentration of [H+] is 10-2mole/L or 0.01M
You’re still talking crazy! That number is small!
It’s not a small number. Remember that a mole is 6.022 X 1023. That’s a very large number! Think about it! A solution of pH 4 is acidic, but if we plug in the formula, we realize that this is equal to 0.0001M H+ – less than pH 2 at 0.01M!
But let’s compare it to the [H+] content of H2O. Now I’m going to sound crazier! Water can be thought of as being in an equilibrium where some of the molecules are ionizing and deionizing. We can express this in 2 ways:
- H2O ⇋ H+ + OH–
- 2H2O ⇋ H3O+ + OH–
So at any given point, a liter of H2O at neutral pH (7) has 10-7 moles of H+ ions. Incidentally, it also has 10-7 moles of OH– in solution. The second expression indicates the formation of a hydronium ion (H3O+) instead of a free proton in solution. So something that is pH 2 is a stronger acid than pH 4, right? Nope. That just indicates the amount of free protons in solution. It is more acidic but acid strength means something else. When we talk about strong acids, it means that it is more likely to donate a proton to the solution because it is more likely to ionize. Let’s look at the following:
- HA ⇋ H+(aq) + A–(aq) Where HA is an acid dissociating in solution
If this dissociation is very high, then we say that it is a strong acid. Similarly, a compound like NaOH readily dissociates completely in solution and provides OH– ions that can readily remove H+ from solution –a strong base! We speak of dissociation in terms of rates and we express this as the acid dissociation constant, Ka. This is calculated using the concentrations of [H+] (proton), [A–] (conjugate base) and [HA] (non-dissociated) at equilibrium:
- Ka = ([H+][A–])/[HA]
Just like the orders of magnitude we have when discussing pH, the rates of dissociation are more conveniently communicated on a logarithmic scale.
- pKa = -Log10Ka
Think about it this way, if the concentration of the dissociated ions is very high, the numerator in the rate is very high → Ka is great. In other words, at equilibrium, the dissociation reaction looks more unidirectional than bi-directional as the compound is readily ionized:
- HA → H+(aq) + A–(aq)
On this scale, we refer to anything with a pKa < -2 as a strong acid since it will readily dissociate in solution. This form of the dissociation constant is extremely useful in estimating the pH of buffer solutions and for finding the equilibrium pH of the acid-base reaction (between the proton and the conjugate base). We can estimate the pH by utilizing the Henderson-Hasselbalch Equation:
- pH = pKa + Log10([A–]/[HA])
Surface Tension
Polar Covalent Bonds
H2O is a polar covalent molecule. The Bonds between the H atoms and the O atom arise from sharing electrons. These shared electrons form to satisfy the octet rule. However, Oxygen is a “selfish” sharer. The electronegative aspect of Oxygen means that the electrons of the H2O molecule preferentially associate near the Oxygen atom, creating partial charges. We indicate this by placing a δ- near the O and δ+’s near the H atoms. These partial charges make the H2O polar. Because of this polarity, H2O molecules arrange in a highly structured way.
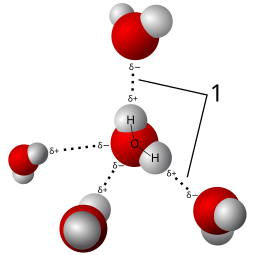
These weak associations that arise from the polar:polar attractions are referred to as Hydrogen Bonds (H-bonds). While independently weak, the summation of all the H-bonds are very strong. These associations give rise to the special properties of water: surface tension, cohesion, adhesion, high specific heat capacity.
Surface Tension
Surface tension presents as an invisible film that encompasses the surface of water. The attractive forces arising from the intermolecular cohesion holds the surface of water together.

This paperclip would sink if it broke through the surface of the water

This water strider is not on top of the water because it is light. It has not broken through the surface of the water and is therefore, on top of the water.
Solutions
Water is an excellent solvent of other polar compounds. Table salt (NaCl) ionizes readily in water. The δ- O associate around Na+ while the δ+ H associate with the Cl–. If NaCl is dissolved in H2O, what do you think happens to the intermolecular interactions between water molecules? What do you think would happen to the H-bonds? Would you expect there to be a difference in the surface tension? How do you think this explains the difference of boiling or freezing?
Running on Water
Lizards of the genus Basiliscus have the nickname “Jesus Christ lizard” for their very special adaptation regarding water.

Basiliscus plumifrons (green basilisk)

Basiliscus basiliscus (common basilisk)
In the face of danger, these lizards run on their hind legs across water to escape predation. Their hind legs have long toes that help in increasing surface area to distribute their weight so they can propel themselves on the surface of the water. They do not sink because the surface tension of the water is not broken by the large surface covered by their feet. After about 4.5m, they lose sufficient momentum to propel themselves on the water surface and break through. H-bonds enable this adaptation.
Video from National Geographic
What if?
The namesake of these lizards walked on water but was also able to turn water into wine. Wine is a solution of ethanol (11%) in water. Ethanol has a polar end and a non-polar end.
- How successful would the basilisk run on wine?
- Why?
- How could you test this without necessarily using a lizard?
Comparing Forms
Comparative Anatomy
Georges Cuvier was a French naturalist and zoologist who utilized the idea of comparative anatomy to establish the field of paleontology. He initially observed that features found in fossils could indicate shared function and relatedness between these features. He illustrated that African and Asian elephants represented 2 distinct species. Furthermore, he identified mammoth fossils as distinct separate species from living elephants.
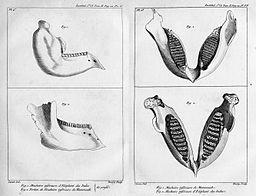
Cuvier supported the idea of special creation to describe the origins of all life on Earth and harshly criticized transmutation as a means for various life forms to exist. Despite this, his use of comparative anatomy later provided crucial evidence that supported transmutation. Specifically, the ideas of homologous and analogous structures. With homologous structures, similar structures appear between similar species despite function and overall structure of those parts. With analogous structures, similar structures reflect a shared function due to the environment as opposed to a shared ancestry. This similarity of structure due to function and environment is referred to as convergence.

The similarity between the gross structure of the bodies of sharks and dolphins is related to the aquatic environment, but their fins are derived from disparate structures.
The case of 2 skulls
Two skulls are presented below. Both skulls have similar features.

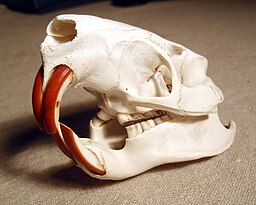
Both skulls show very large teeth at the front of the mouth followed by a handful of flat grinding teeth in the rear of the mouth. Overall structure of these two skulls is very similar. To the untrained eye (and without any size reference) we could assume that they might even be from the same species.
- Are these 2 skulls the same species of organism?
- What are the supporting features that indicate that they belong to the same species?
- If we look carefully at the dentition (teeth), do we learn about the the relatedness of the skulls?
- What might we assume about diet by looking at the teeth from these skulls? Describe the teeth and identify what they would be used for.
What we notice immediately are the very large teeth at the front of the skulls. We might also notice further back in the mouth, the presence of very flat looking teeth.
Adaptation
The teeth from the 2 skulls indicate that they might be specialized for gnawing. In fact, the 2 skulls are clearly classifiable as rodents!
- Compare the 2 teeth in the front of your mouth (incisors) to the ones in the skulls
- While they are greatly different in size, do these teeth have a similar function?
As it turns out, the left skull actually belongs to a beaver and right skull belongs to a nutria.
Beavers are anthropomorphically characterized as industrious. They are large, semi-aquatic rodents that are known for their behavior of building dams in wetlands. Nutria are also semi-aquatic rodents, but they are smaller than beavers. While nutria have some similar features to beavers, nutria have long rat-like tails instead of the long flat paddled tail of the beaver.


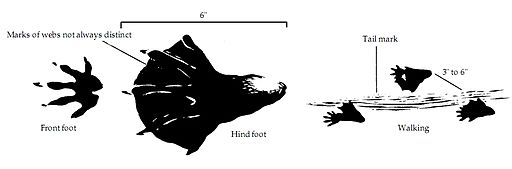
Comparative anatomy using fossils is limited by the fact that soft tissue doesn’t fossilize readily. Looking at the living organisms, we can see that both species have webbed hind feet.
- Is this a common feature to all rodents?
- Swans also have webbed feet. Does this mean they are closely related?
- What does it mean if we decide that swans are not closely related?
Beavers occur naturally in the northern hemisphere while Nutria occur naturally in the southern hemisphere. Just like another invasive rodent species, nutria also exist in North America because they were introduced here.
- If beavers and nutria have similar structure and adaptations to a specific environment, would we expect them to compete?
- Would you expect the beaver to succeed in the natural environment of the nutria?