Table of Contents
Why Are Cells Small?
Overview
In this activity, you will use agar blocks to represent cells of different sizes to explore how materials diffuse. Cells remain small to maintain a high surface area to volume ratio. This ratio allows for materials to be quickly moved in and out of the cell.
Materials
Each lab group will need the following materials:
- 3 blocks of agar (1 cm, 2 cm, and 3 cm)
- ruler
- 1 beaker
- 200 ml NaOH
- paper towels
- knife
Method, Part 1
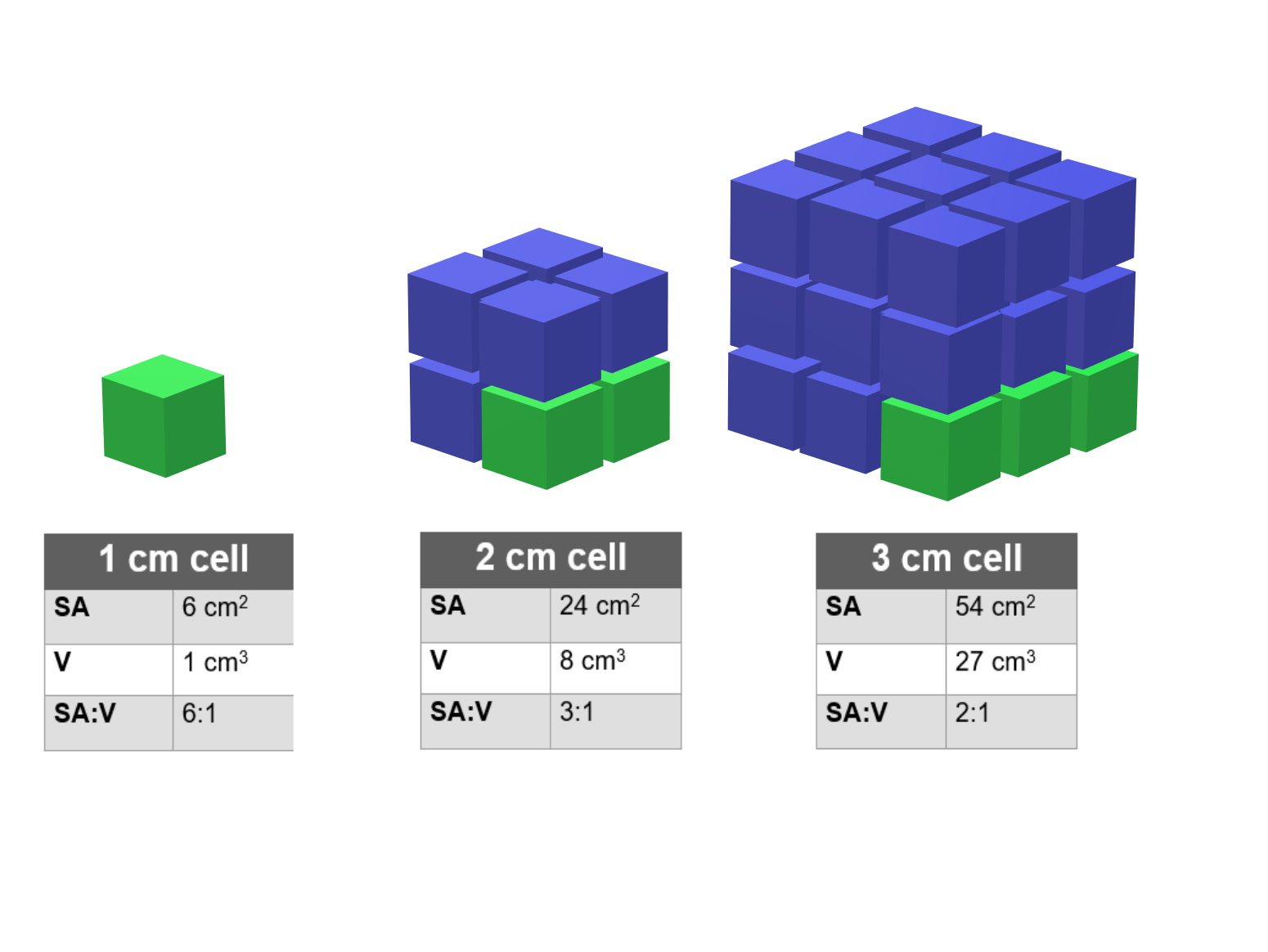
- Take 3 blocks of agar of different size (1cm, 2cm, 3cm). These are our cell models.
- Measure the length, width and height of each cube using a ruler.
- Calculate the area of each face of the cubes and add all the areas together for a single cube.
- A cube has 6 faces, so the total surface area is the same as the area of one side multiplied by 6.
- Calculate the volume of each cube.
- Report the surface area-to-volume in the table below.

Before You Continue
Discuss the following with your lab group:
- Which cube has the greatest surface area to volume ratio?
- Which cube has the smallest surface area to volume ratio?
- Hypothesize: In an osmosis or diffusion experiment, which cube size would have the greatest diffusion rate?
Method, Part 2
- Place cubes into a beaker and submerge with 200 ml NaOH.
- Let the cubes soak for approximately 10 minutes.
- Periodically, gently stir the solution, or turn the cubes over.
- After 10 minutes, remove the NaOH solution.
- Blot the cubes with a paper towel.
- Promptly cut each cube in half and measure the depth to which the pink color has penetrated. Sketch each block’s cross-section in your notebook.
- Record the volume that has remained white in color.
- Do the following calculations for each cube and complete the following data table:

Conclusion
- Which cube had the greatest percentage of diffusion?
- Did this meet your expectations with your hypothesis?
- If you designed a large cell, would it be a large sphere or something long and flat?



