Table of Contents
Detection of Carbohydrates (Starch)
Overview
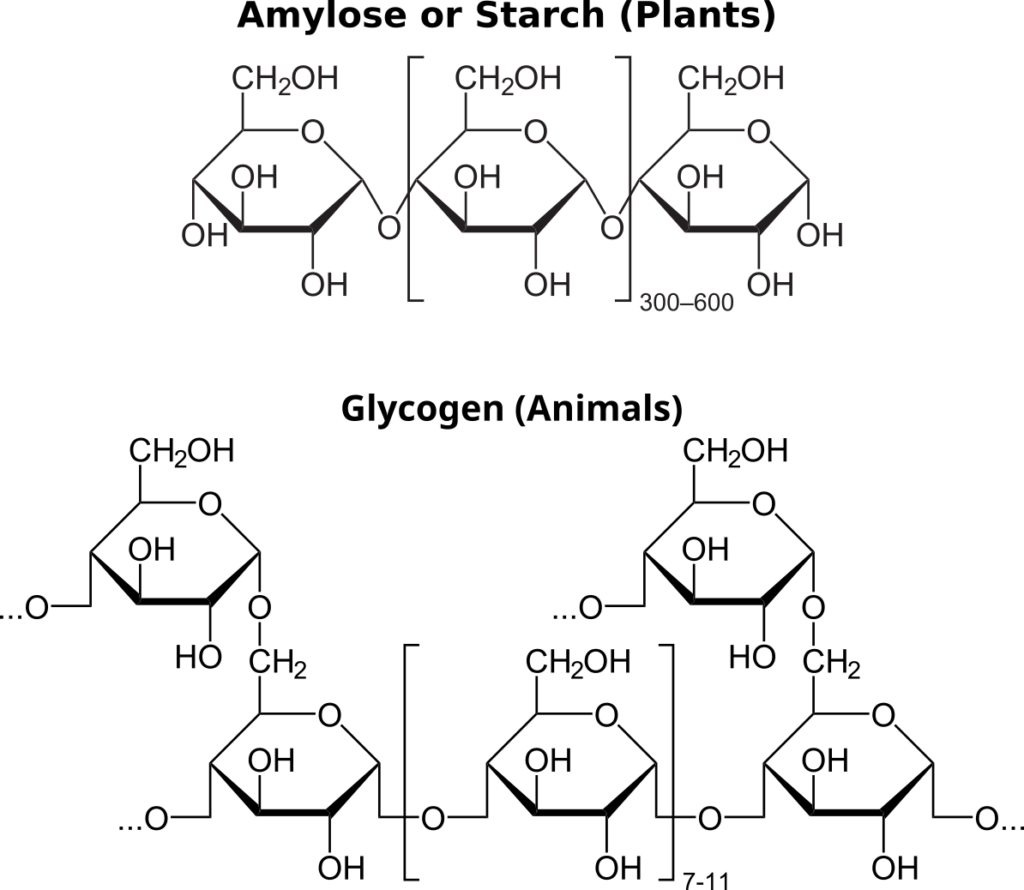
Carbohydrates that are used for energy storage are not reducing sugars since they are polymers that lack free aldehydes. Plant cells store energy in the form of starches like amylose or pectin. Since these molecules are larger than monosaccharides or disaccharides, they are not sweet to the taste and are not very soluble in water.
Iodine (iodine-potassium iodide, I2KI) staining distinguishes starch from monosaccharides, disaccharides, and other polysaccharides. The basis for this test is that starch is a coiled polymer of glucose — iodine interacts with these coiled molecules and becomes bluish black. Iodine does not react with other carbohydrates that are not coiled, and remains yellowish brown. Therefore, a bluish-black color is a positive test for starch, and a yellowish-brown color (i.e., no color change) is a negative test for starch. Notably, glycogen, a common energy storage polysaccharide in animals, has a slightly different structure than does starch and produces only an intermediate color reaction.
Materials
Each lab group will need the following materials:
- reducing sugar solution
- glucose solution
- sucrose solution
- starch solution
- distilled water
- potato juice
- apple juice
- iodine
- 7 test tubes
- test tube rack
- wax pencil
Method
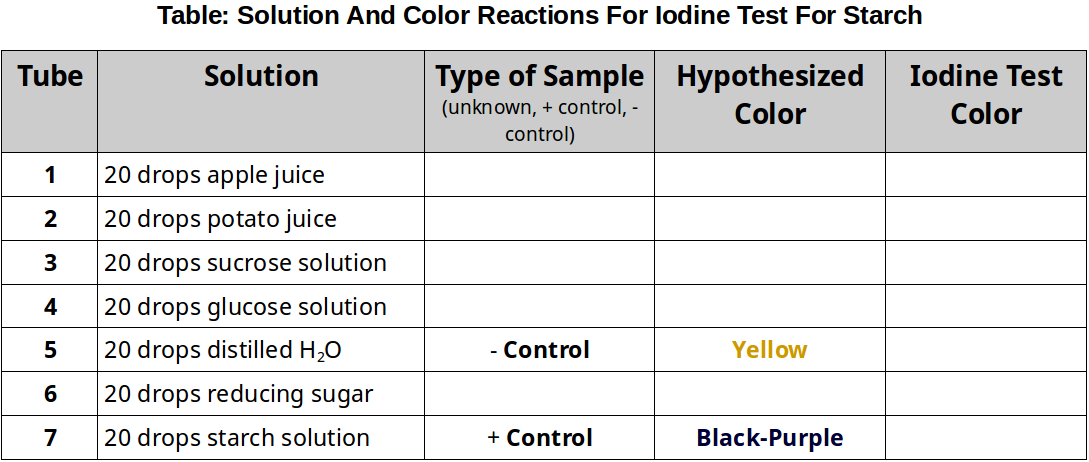
- Obtain 7 test-tubes and number them 1-7 with the wax pencil.
- Use the information in the table below to fill each test tube with the sample solutions.
- Your instructor may ask you to test some additional materials. If so, include additional numbered test tubes.
- Indicate in the table below whether the the sample you are testing is a positive control, a negative control, or an unknown.
- Predict the color change (if any) for each sample (use the sample type to aid in your prediction).
- Add 10 drops of iodine to each tube. This test does NOT require boiling.
- Record the color of the tubes’ contents in the table below.
Note: You will need these results for Lab 5.
Print this page


