Table of Contents
Energy and Catalysts
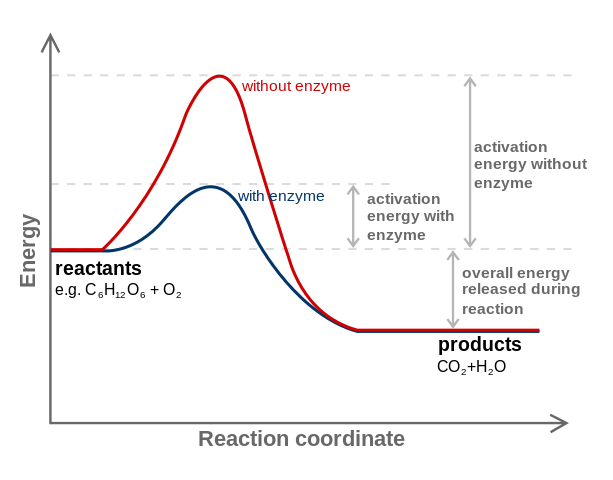
In biological systems, energy is roughly defined as the capacity to do work. Molecules are held together by electrons in bonds. Breaking and building these bonds requires an input of energy. The energy needed to initiate such reactions is referred to as activation energy (EA). Sometimes the necessary energy to initiate a reaction is so great, that it greatly limits the likelihood of the reaction ever occurring. Catalysts are chemicals that take part in facilitating reactions by reducing the energy of activation. If the activation energy is reduced, the likelihood of a reaction occurring is greatly enhanced. In cells, the catalysts are often made of proteins and called enzymes.
Mechanisms of Enzyme Action
Reactants in enzymatic reactions are called substrates. They have an imperfect fit to a binding domain of the enzyme called the active site. Substrate binding to this active site induces a change in the shape of the protein that coordinates the substrate into a transition state that will reduce the amount of EA required for the reaction to go to completion. The induced fit of the protein also aids in coordinating other cofactors or coenzymes that will aid in the reaction.
The reaction follows the standard flow where the enzyme (E) and the substrate (S) interact to form an enzyme-substrate complex (ES). The ES then dissociates into enzyme and the resultant product (P)
E + S ⇒ ES ⇒ E + P
The induced fit of the enzyme-substrate complex coordinates the transition state to facilitate the reaction. This induced fit occurs through non-covalent means that result in a tugging on the molecules (an application of energy) while molecules are coaxed into the reactions.
Coenzymes can be covalently linked to amino acid side chains of the enzyme and are also referred to as prosthetic groups. While prosthetic groups are organic in nature, they may also involve the coordination of metal ions, like the heme group which binds to iron. These prosthetic groups enhance the repertoire of the amino acids to provide additional functioning to the entire protein. Early coenzymes were described as being vital to normal functioning and were characterized as organic molecules with amine groups. Because of this coincidence, they were referred to as vitamins (for vital amines) though not all vitamins have amine groups. The trace metal ions that work with these groups are also required and represent the minerals on food items.
The Affect of Environmental Factors on Enzyme Activity
Enzymes are proteins, so they are subject to denaturation. When enzymes denature, the active site loses its specific shape. This results in the inability of the substrates to properly bind to the active site, decreasing enzyme activity. Denaturation can be triggered by pH values or temperatures outside of the enzyme’s optimum range. This range is determined by the environment in which the enzyme normally functions. For example, the enzyme pepsin has an optimum pH of around 2 because it functions in the acidic stomach to digest protein.
Print this page


