Polar Covalent Bonds
H2O is a polar covalent molecule. The Bonds between the H atoms and the O atom arise from sharing electrons. These shared electrons form to satisfy the octet rule. However, Oxygen is a “selfish” sharer. The electronegative aspect of Oxygen means that the electrons of the H2O molecule preferentially associate near the Oxygen atom, creating partial charges. We indicate this by placing a δ- near the O and δ+’s near the H atoms. These partial charges make the H2O polar. Because of this polarity, H2O molecules arrange in a highly structured way.
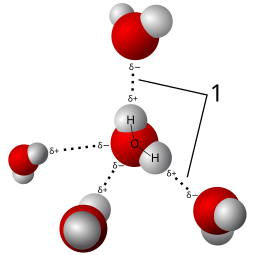
These weak associations that arise from the polar:polar attractions are referred to as Hydrogen Bonds (H-bonds). While independently weak, the summation of all the H-bonds are very strong. These associations give rise to the special properties of water: surface tension, cohesion, adhesion, high specific heat capacity.
Surface Tension
Surface tension presents as an invisible film that encompasses the surface of water. The attractive forces arising from the intermolecular cohesion holds the surface of water together.

This paperclip would sink if it broke through the surface of the water

This water strider is not on top of the water because it is light. It has not broken through the surface of the water and is therefore, on top of the water.
Solutions
Water is an excellent solvent of other polar compounds. Table salt (NaCl) ionizes readily in water. The δ- O associate around Na+ while the δ+ H associate with the Cl–. If NaCl is dissolved in H2O, what do you think happens to the intermolecular interactions between water molecules? What do you think would happen to the H-bonds? Would you expect there to be a difference in the surface tension? How do you think this explains the difference of boiling or freezing?
Running on Water
Lizards of the genus Basiliscus have the nickname “Jesus Christ lizard” for their very special adaptation regarding water.

Basiliscus plumifrons (green basilisk)

Basiliscus basiliscus (common basilisk)
In the face of danger, these lizards run on their hind legs across water to escape predation. Their hind legs have long toes that help in increasing surface area to distribute their weight so they can propel themselves on the surface of the water. They do not sink because the surface tension of the water is not broken by the large surface covered by their feet. After about 4.5m, they lose sufficient momentum to propel themselves on the water surface and break through. H-bonds enable this adaptation.
Video from National Geographic
What if?
The namesake of these lizards walked on water but was also able to turn water into wine. Wine is a solution of ethanol (11%) in water. Ethanol has a polar end and a non-polar end.
- How successful would the basilisk run on wine?
- Why?
- How could you test this without necessarily using a lizard?




The basilisk would run very well on wine because there would be even more polar:polar bonds than in water. You could test this by finding some object with the surface area similar to that of a basilisk’s foot and seeing if it floats on the wine.