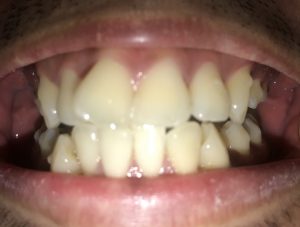
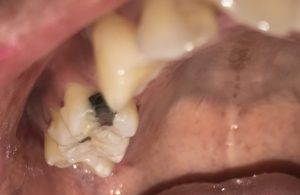
SRP Pre-Treatment – April 09, 2019
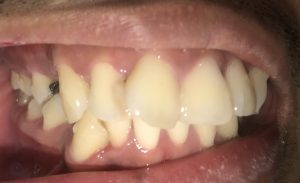
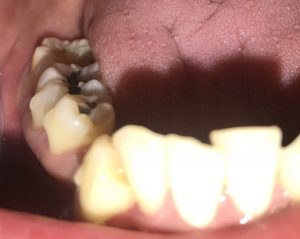
SRP Post Treatment – April 09, 2019
Assessment
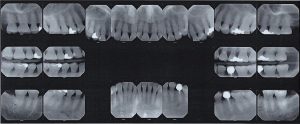
The patient is a 27 year old, Asian male. The patient reported his last dental services, including prophylaxis and x-rays were performed in 2012. He reported smoking about 10 cigarettes per week and electronic cigarettes daily. Dental examination and periodontal charting was performed during his SRP treatment in April 2019.
Diagnosis of Oral Conditions and Planning
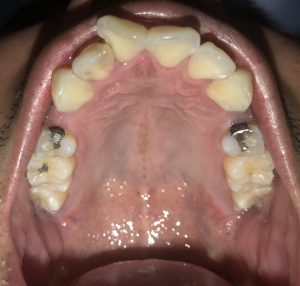
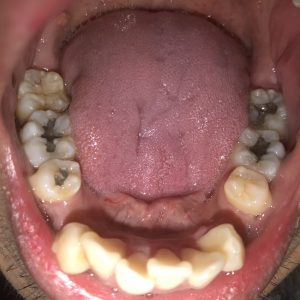
A white, raised 1 x 1 clustering lesion was present on the patient’s soft palate when performing the intra-oral inspection. Referral was given for Oral Surgery evaluation and biopsy. The patient presented with multiple restorations, but no active carious lesions. Referrals were given for defective or broken margins of amalgam and composite fillings. The periodontal assessment for the patient classified him as Periodontal type I, localized type II in the posterior region as the patient had 4-7mm PD, radiographic horizontal boneloss, and heavy BUP. The patient was a heavy case value as there was moderate – heavy supragingival calculus on the lower anterior, heavy generalized subgingival calculus interproximally, and moderate biofilm present. The patient was evaluated and treated using radiographs, dental and periodontal assessments, and had SRP treatment performed in two visits. Sodium Fluoride varnish 5% was placed post SRP treamtent. The patient was recommended to return for recall every 3 months.
Implementation
The patient had UR and LR quadrants scaled at his first visit and at his follow up appointment, a week later, the UL and LL were scaled to completion. After SRP was performed, the UR was re-evaluated and Arestin was placed on the right side as the patient’s pocket depths had not reduced. The following teeth were treated with Arestin and the patient will return for re-evaluation of pocket depths:
- #2 MB – 5mm
- #3 MB – 5mm
- #3 DL – 5mm
- #4 DB – 5mm
- #29 DL – 5mm
- #30 ML – 6mm
- #30 DL – 6mm
- #31 MB – 6mm
Evaluation & Documentation
Follow-up Arestin evaluation scheduled for May 15, 2019.