Table of Contents
Solutions
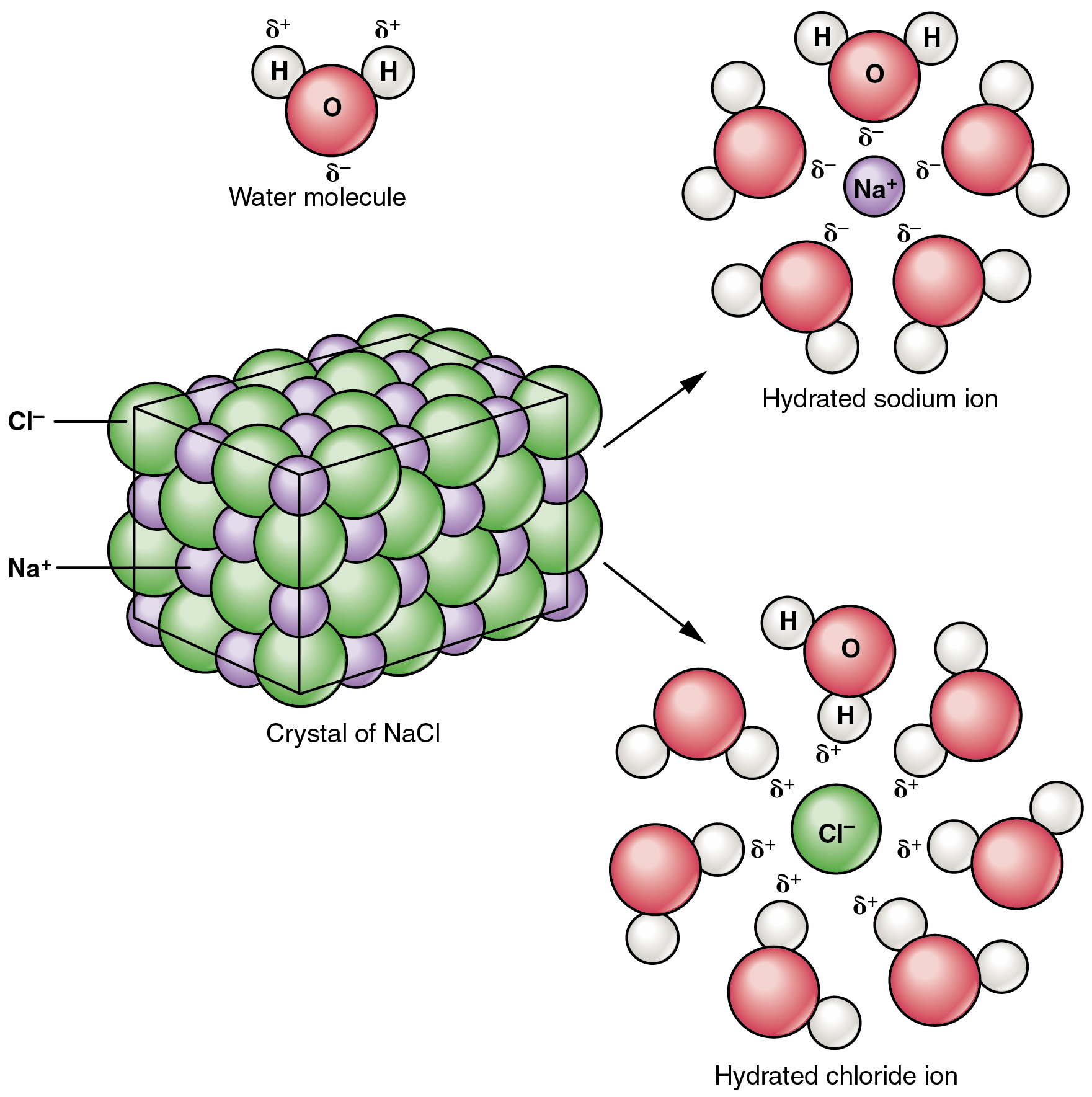
Water is an excellent solvent of other polar compounds. Table salt (NaCl) ionizes readily in water. The δ– O associate around Na+ while the δ+ H associate with the Cl–. If NaCl is dissolved in H2O, what do you think happens to the intermolecular interactions between water molecules? What do you think would happen to the H-bonds? Would you expect there to be a difference in the surface tension? How do you think this explains the difference of boiling or freezing?
Dissociation of water
In pure water, there is always a small percentage of water molecules in solution that dissociate (or ionize) into equal amounts of hydrogen ions (H+) and hydroxide ions (OH–).
In solution, water molecules move freely and some bump on each other, exchanging protons and electrons.
Concentration of H+ ions in pure water = 10-7 moles/L
Concentration of OH– ions in pure water = 10-7 moles/L
These ions, OH– and H+, (while very rare) are EXTREMELY important. They are very reactive and establish acid-base reactions in aqueous solutions. Changes in concentrations of H+ and OH– can drastically affect the chemistry of a cell.
pH and the pH scale

In aqueous solutions, adding certain solutes, called acids and bases, modifies the concentrations of H+ and OH–
Biologists use something called the pH scale to describe the concentration of these ions in solution.
The pH of a solution is inversely dependent on H+ ions:
pH = -log[H+] (in M) AND…
[H+] x [OH–] = 10-14 M (M = mol/L)
Acid rain
Acid rain is formed when sulfur dioxide (SO2) and nitrogen oxides (NOx) are emitted by anthropogenic and natural sources and transported by the air current. They react with water and oxygen in the atmosphere to form more acidic pollutants. They fall to the ground by wet precipitation when associated with rain and by dry deposition when associated to particles and gas. Acid rain harms humans, vegetation and wildlife.
Learn more about acid rain.