Table of Contents
What are lipids?
Lipids are the class of macromolecules that mostly serve as long-term energy storage. Additionally, they serve as signaling molecules, water sealant, structure and insulation. Lipids are insoluble in polar solvents such as water, and are soluble in nonpolar solvents such as ether and acetone.
You can see in the figure that lipids contain chains and rings of hydrocarbons, and some functional groups.
Fats and triglycerides
Fats or triglycerides are made of glycerol and three fatty acid chains. They form through 3 dehydration synthesis reactions between a hydroxyl of the glycerol and the carboxyl group of the fatty acid. Fatty acids tend to be chains of 16-18 carbons long.
Saturated vs unsaturated fats
Saturated fats do not contain carbon-carbon double bond. They are saturated in hydrogen atoms. This means that each atom of carbon is bound to maximum number of hydrogens. The molecule takes up little space in three dimensions. Many molecules can stack upon each other. Saturated fats are solid at room temperature.
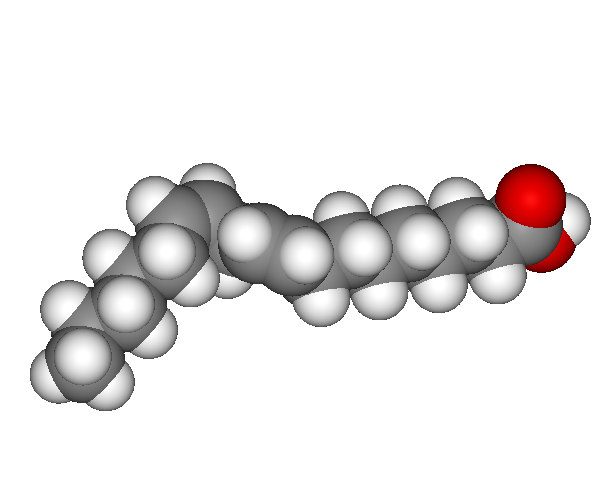
A polyunsaturated fatty acid contains more than one carbon-carbon double bond or unsaturation. Since carbon makes two bonds with the other carbon, it carries less hydrogen atoms. A kink from the double bond increases the amount of three dimensional space that the molecule fills. Unsaturated fats tend to be liquid at room temperature.
Trans fatty differ from cis fatty acids because of the position of the hydrogen atoms towards the carbon-carbon double bond.
Trans fat are harmful and must be limited in a diet because it increases the risk of heart diseases. Despite an unsaturated bond, the molecule fills as much space as a saturated fatty acid and is solid at room temperature. Trans fats usually arise from artificial saturation techniques.