Table of Contents
Structure of Lipids
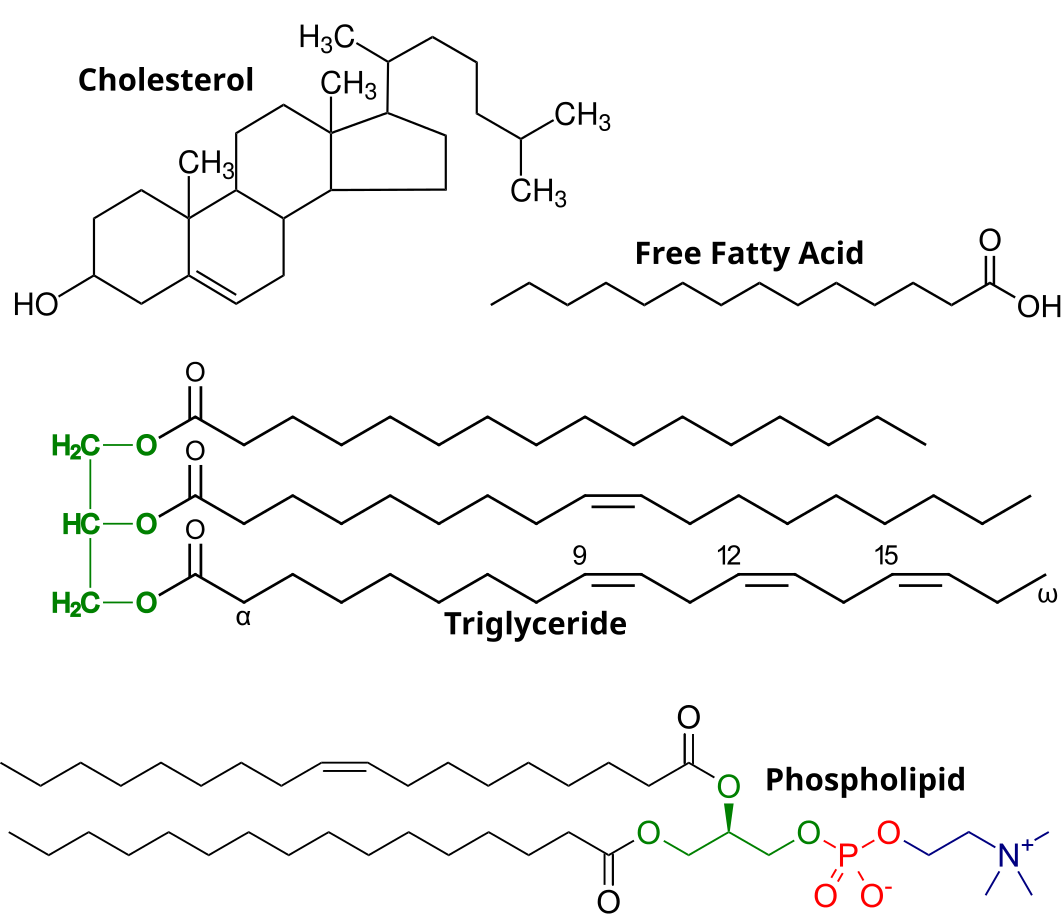
Lipids are the class of macromolecules that mostly serve as long-term energy storage. Additionally, they serve as signaling molecules, water sealant, structure and insulation. Lipids are insoluble in polar solvents such as water, and are soluble in nonpolar solvents such as ether and acetone.
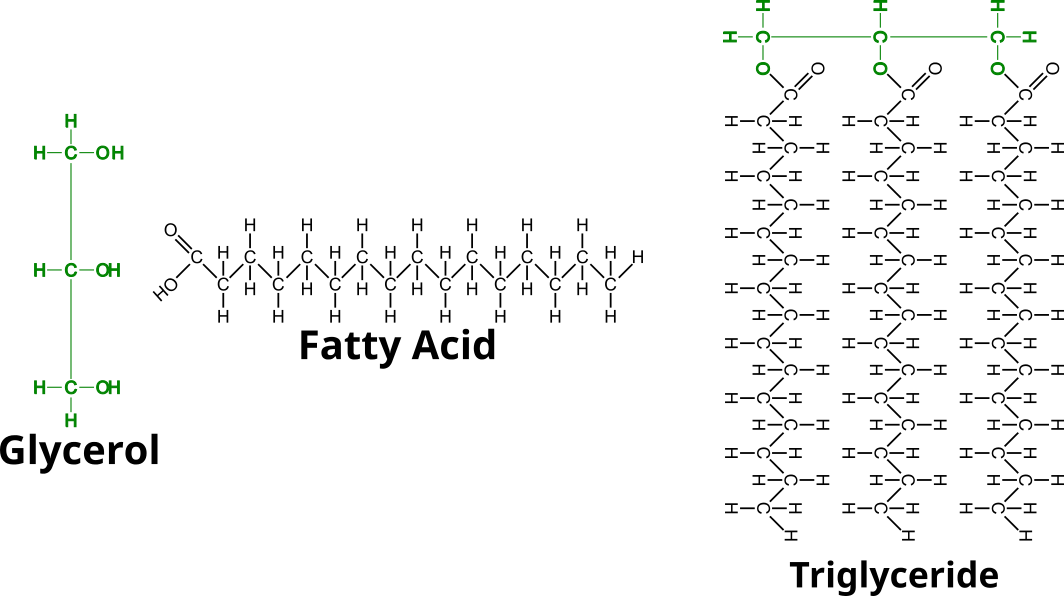
Fats or triglycerides are made of glycerol and three fatty acid chains. They form through 3 dehydration synthesis reactions between a hydroxyl of the glycerol and the carboxyl group of the fatty acid.
Saturated vs. Unsaturated Fats
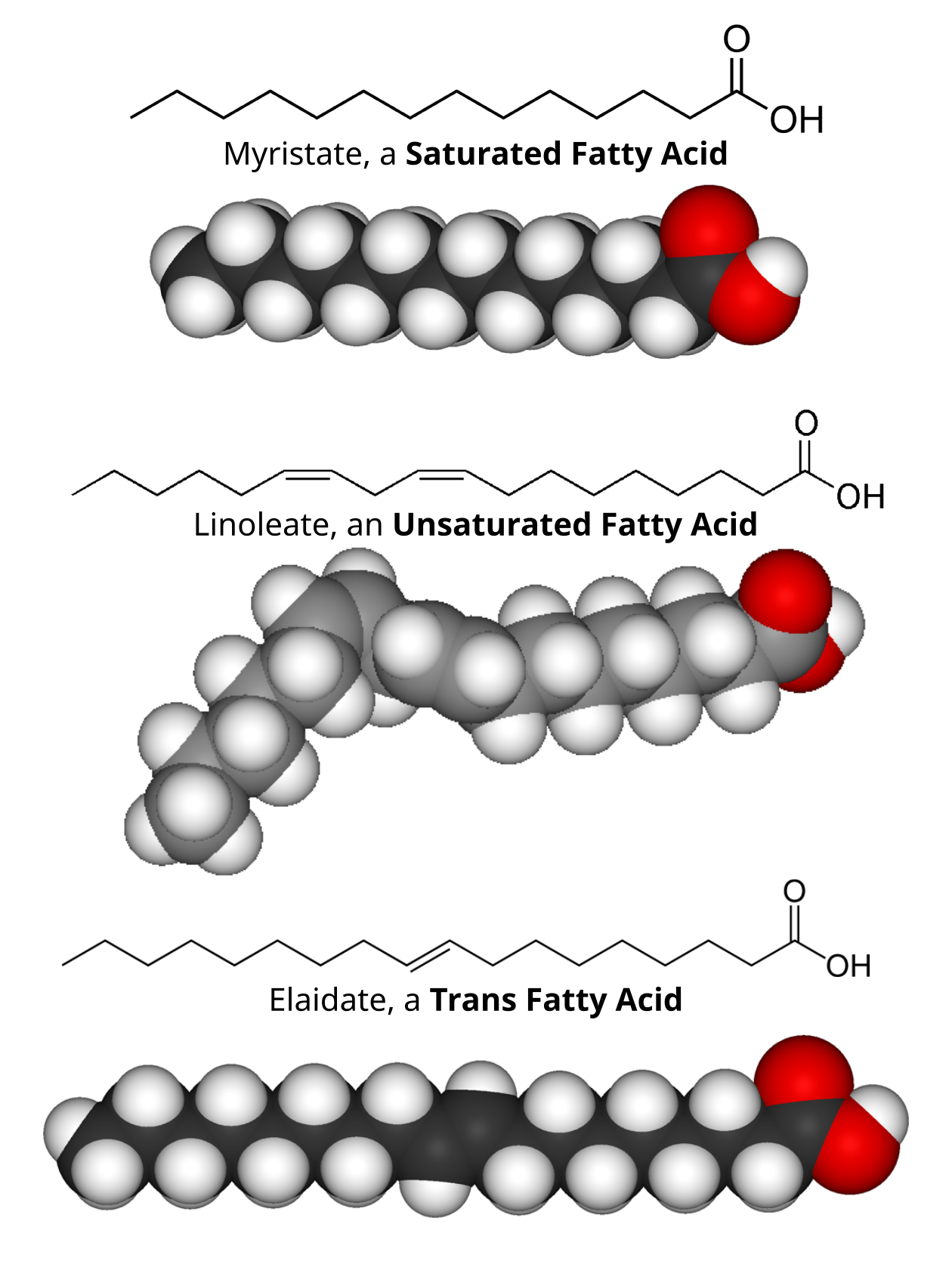
Saturated fatty acids contain single bonds between their carbon atoms. The molecule takes up little space in three dimensions. Many molecules can stack upon each other. Saturated fats are solid at room temperature.
Unsaturated fatty acids contain at least one double bond between two carbon atoms. A kink from the double bond increases the amount of three dimensional space that the molecule fills. Polyunsaturated fatty acids contain more than one double bond. Unsaturated fats tend to be liquid at room temperature.
Trans fatty acids usually arise from artificial saturation techniques. Despite an unsaturated bond, the molecule fills as much space as a saturated fatty acid and is solid at room temperature.