Table of Contents
Detection of Proteins
Overview
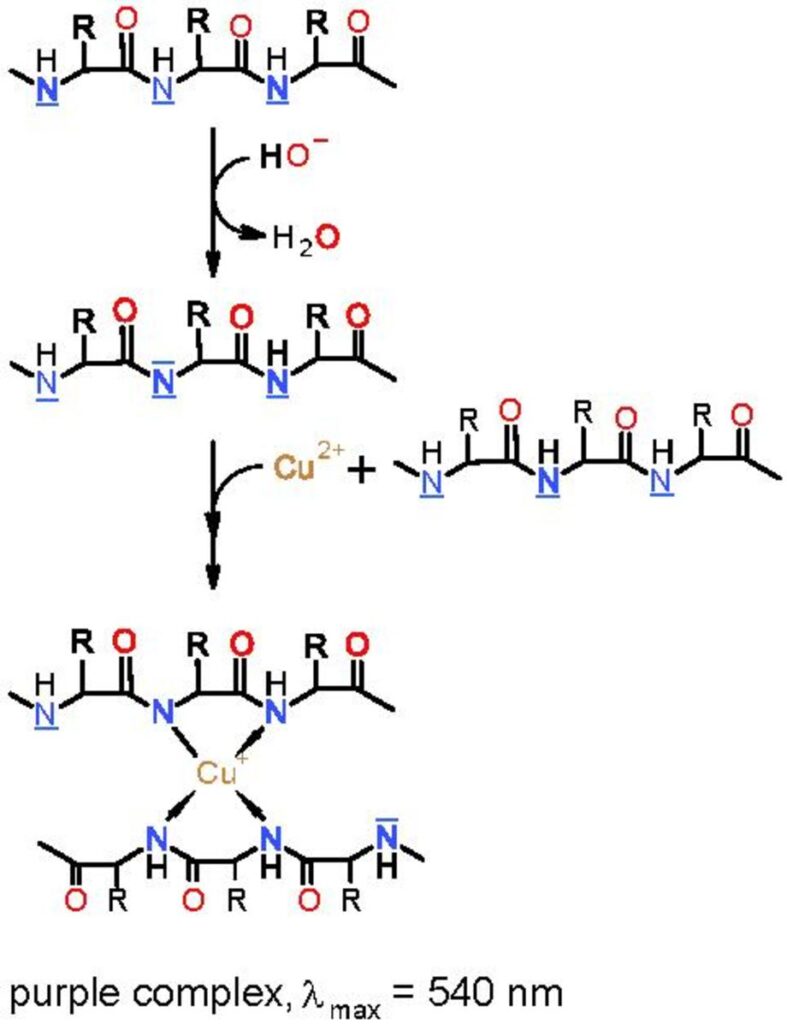
In this activity, you will test a variety of samples to see if they contain proteins. Proteins can be detected through the use of the biuret test. Specifically, peptide bonds (C-N bonds) in proteins complex with Cu2+ in biuret reagent and produce a violet color. A Cu2+ must complex with four to six peptide bonds to produce a color; therefore, free amino acids do not positively react. Long polypeptides (proteins) have many peptide bonds and produce a positive reaction to the reagent. Biuret reagent is an alkaline solution of 1% CuSO4, copper sulfate. A violet color is a positive result for the presence of protein, and the intensity of color is proportional to the number of peptide bonds in the solution.
Materials
Each lab group will need the following materials:
- protein solution
- amino acid solution
- egg albumin
- distilled water
- urine sample 1
- urine sample 2
- biuret solution
- 6 test tubes
- test tube rack
- wax pencil
Method
- Obtain 6 test tubes and number them 1-6 with the wax pencil.
- Use the information in the table below to fill each test tube with the sample solutions.
- Indicate in the table below whether the the sample you are testing is a positive control, a negative control, or an unknown.
- Predict the color change (if any) for each sample (use the sample type to aid in your prediction).
- Formulate a hypothesis about the components of the unknown solutions.
- Add 3 drops of biuret reagent (1.0% CuSO4 with NaOH) to each tube and mix.
- Record the color of the tubes’ contents in the table below.

Conclusions About Urine Samples
Using the data from the detection of reducing sugars in Lab 4 and the detection of protein, what can you conclude about the two urine samples?
Print this page