Table of Contents
Chromatography
Overview
In this lab activity, you will use paper chromatography to determine if the food colorings used in colored candy the same as the the FD&C approved chemicals.
Materials
Each lab group will need the following materials:
- 25 cm square piece of chromatography paper
- 4 standard dyes (red, yellow, green, and blue)
- 1 piece of candy (random color)
- ethanol
- mobile phase solution
- 1 flask
- 1 large beaker
- 5 applicator sticks
- pencil
- ruler
- stapler
Procedure
- Obtain a 25 cm square piece of chromatography paper that will fit into the beaker that will serve as the chromatography chamber.
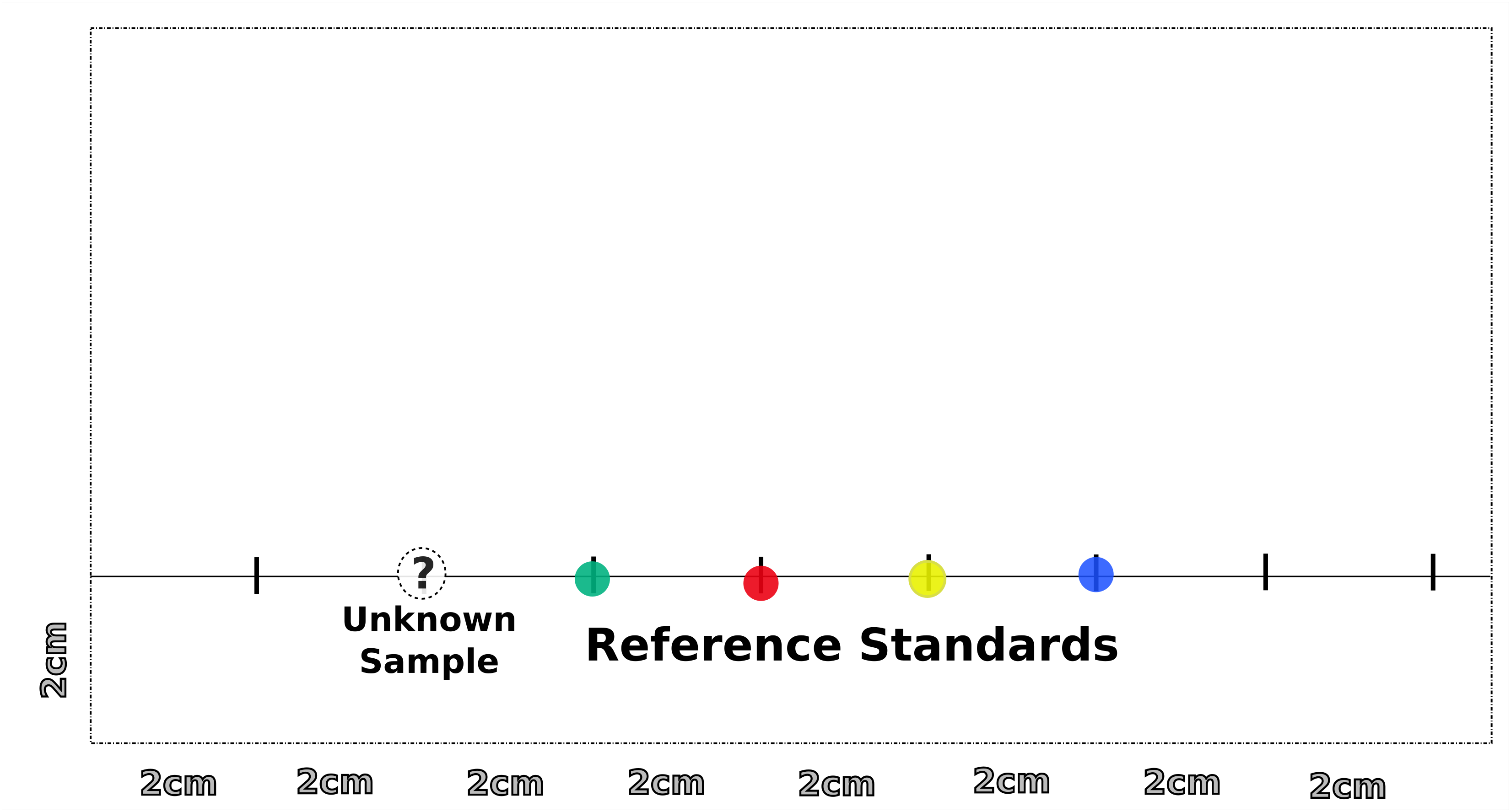
- Draw a pencil line across the lower end of the chromatography paper about 2 cm from the bottom.
- Draw additional vertical tick marks along this line every 2 cm
- Place colored candy in a flask with 2 ml ethanol until the color dissolves into the solution
- Using an applicator, create a very small spot on a tick mark and allow it to dry.
- Repeat application on the spot to make a very small and dark spot.
- Continue to spot reference standards along other tick marks. These reference standards are food coloring.
- Place approximately 1 cm of mobile phase solution (a very polar salt water solution) into the beaker.
- Roll the filter paper into a cylinder and fix with staples.
- Place the cylinder into the beaker and cover for 20 minutes or until the mobile phase reaches 2 cm from the top of the paper.
- Use the pencil to mark the final distance of the mobile phase and allow the paper to dry.
- Once dry, measure the distance of each colored spot from the starting point.
- Each colored spot is a separate analyte.
- Some samples separate into multiple analytes.
- Measure EACH one
- Measure the distance from the starting point to the final point that the solvent reached.
- Calculate Rf values and tabulate results.
Data tables can be found on the next page.
Print this page