Table of Contents
Diffusion
Diffusion is the net movement of a substance from high concentration to low concentration. This difference in concentration is referred to as a concentration gradient. This movement does not require any external energy, but uses the free energy intrinsic to the system.

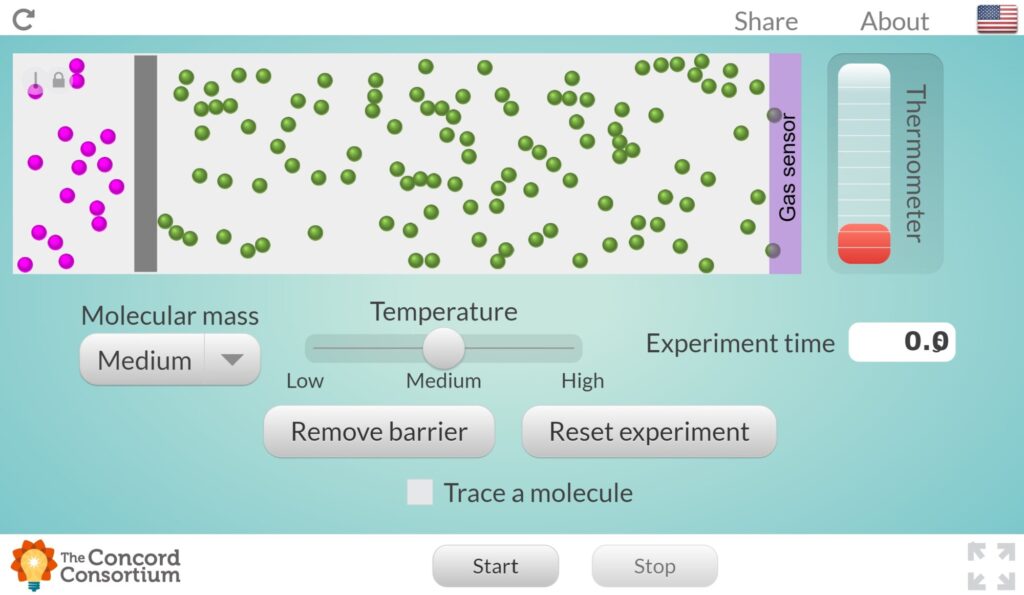
Practice: Exploring the Effects of Temperature and Molecular Mass on Diffusion
Use the link below to explore the ways temperature and molecular mass affect diffusion.
- Use the slider to switch between low, medium, and high temperature. How does this change in temperature affect the movement of the molecules?
- Use the drop-down menu to switch between low, medium, and high molecular mass. How does this change in molecular mass affect the movement of the molecules?
- Based on your observations, what combination of temperature and molecular mass would result in the highest rate of diffusion?
Osmosis
Osmosis is a special case of diffusion. Instead of observing the net change in solute, osmosis follows the net movement of a solvent across a semipermeable membrane. Since a semi-permeable membrane permits specific things to pass through, some solutes are partitioned.
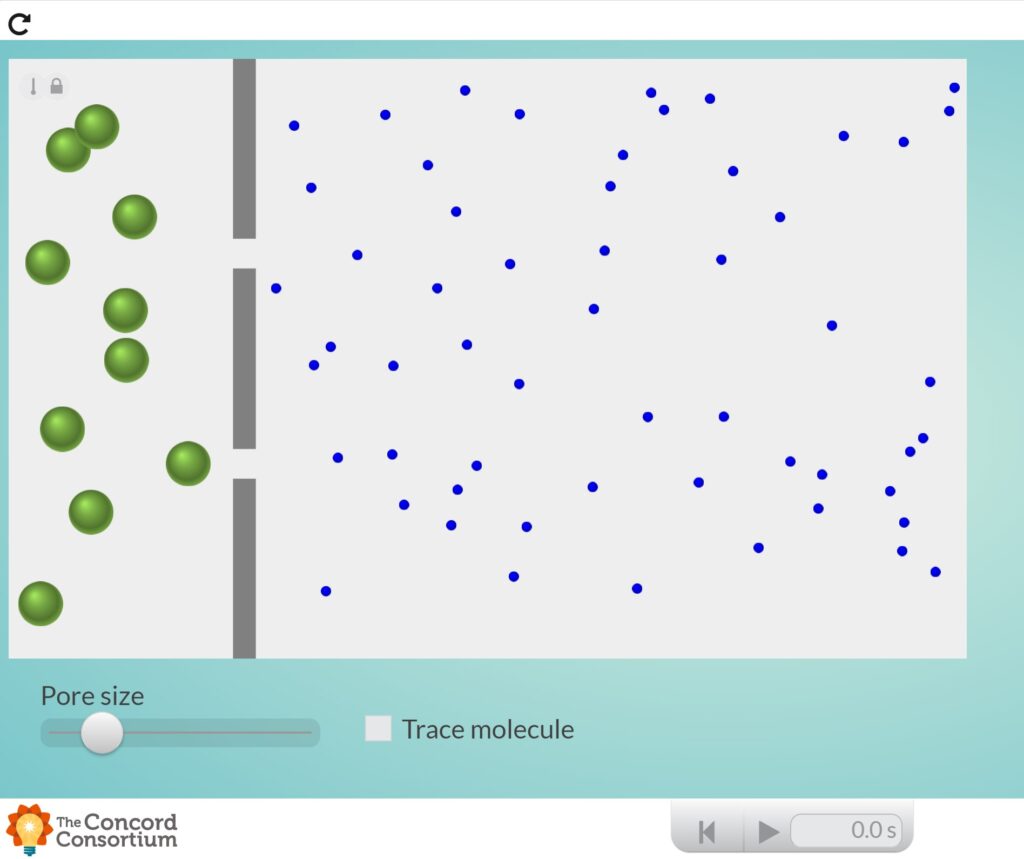
Practice: Explore the Movement of Solute and Solvent Molecules During Osmosis
Use the link below to explore the movement of molecules during osmosis.
- Use the slider to change the size of the pores in the membrane. How does the size of the pores affect the movement of the green solute molecules? How does it affect the movement of the blue solvent molecules?
Tonicity
A cell lacking a cell wall is affected greatly by the tonicity of the environment. In a hypertonic solution where the concentration of dissolved solute is high, water will be drawn out of the cell. In a hypotonic solution where the concentration of dissolved solute is lower than the interior of the cell, the cell will be under great osmotic pressure from the environmental water moving in and can rupture.
Plants have rigid cell walls composed of cellulose. These cell walls permit for maintenance of cellular integrity when the external environment is hypotonic (less dissolved substances). In this situation, the water moves into the cell. Without the cell wall, the cell would burst open from the excessive water pressure entering the cell. This state of swelling is referred to as turgid, resulting from turgor pressure.
When the exterior environment is hypertonic , (greater amount of dissolved substances), the reverse condition occurs whereby the cellular fluid exiting the cell reduces the size of the cytoplasm. This condition is referred to as plasmolysis.
Print this page