Table of Contents
Definition
Beer’s Law is a relationship between the concentration or amount of a dissolved substance in a solution that is reducing the amount of transmitted light due to the absorption of the radiant energy. Lambert’s Law states that the reduction of transmittance was related to the length of the path of light. As the light path increases through a substance, there is a reduction in transmittance. Collectively, these ideas are referred to as Beer-Lambert Law, but most observers will control the path length and simply refer to it as Beer’s Law.
Beer’s Law is based on three factors that affect the absorbance of a solution. The first factor is the concentration of the solution. As the concentration of a solution increases, the amount of light that is absorbed by that solution also increases and the amount of light that is transmitted decreases.
The second factor is the length of the path of light. As mentioned above, as the length of the path of light increases, absorbance also increases and transmittance decreases. This occurs because a longer path of light results in more solution molecules coming into contact with the light and increasing the opportunities for absorption.
The third factor is the molar absorptivity. This is a measure of how well the solution absorbs a particular wavelength of light. The higher the molar absorptivity, the higher the absorbance by that solution.
Beer’s Law can be written as a formula:
A = εbc
where A is the absorbance, ε is the molar absorptivity, b is the length of the light path, and c is the concentration of the solution.
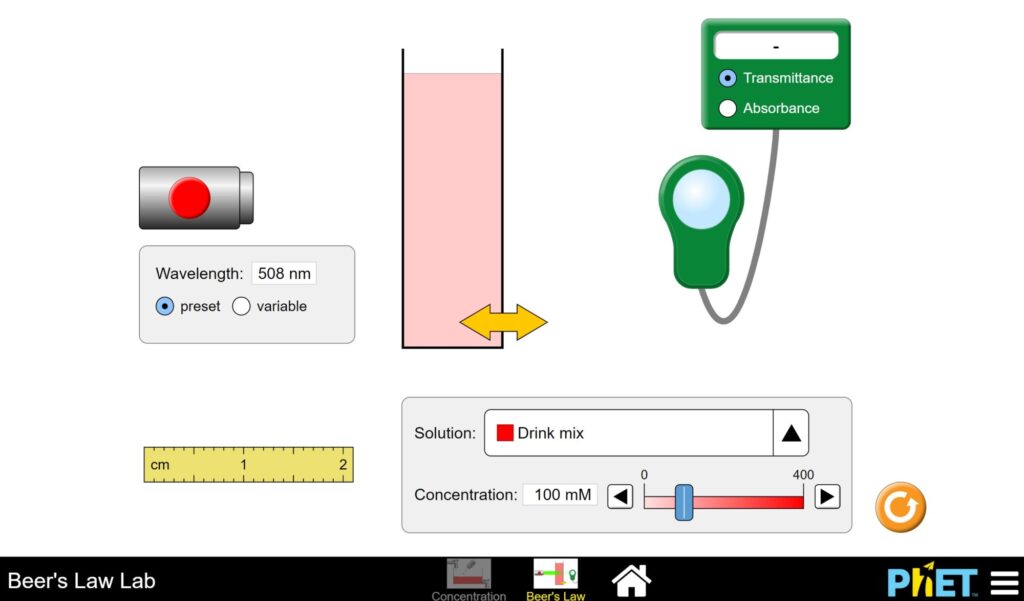
Practice: Exploring Beer’s Law
Launch the simulation and choose Beer’s Law.
Turn on the light source and use the slider at the bottom of the page to increase and decrease the concentration.
- What happens to the contents in the cuvette?
- How does this change the transmittance and absorbance readings?
- Click “variable” and use the slider. What happens to the readings when the wavelength of the laser is a similar color as the solution in the cuvette?
- Consult the color star below and find the color wavelength that is opposite of the color of the solution. Set the laser to this color using “variable” and the slider.
- What is the effect on transmittance and absorbance with this color?
- Using the previous observations (using variable wavelength slider), how would you use the relationship on transmittance/absorbance to best measure the concentration of a solution?