Table of Contents
How Do Atoms Bond?
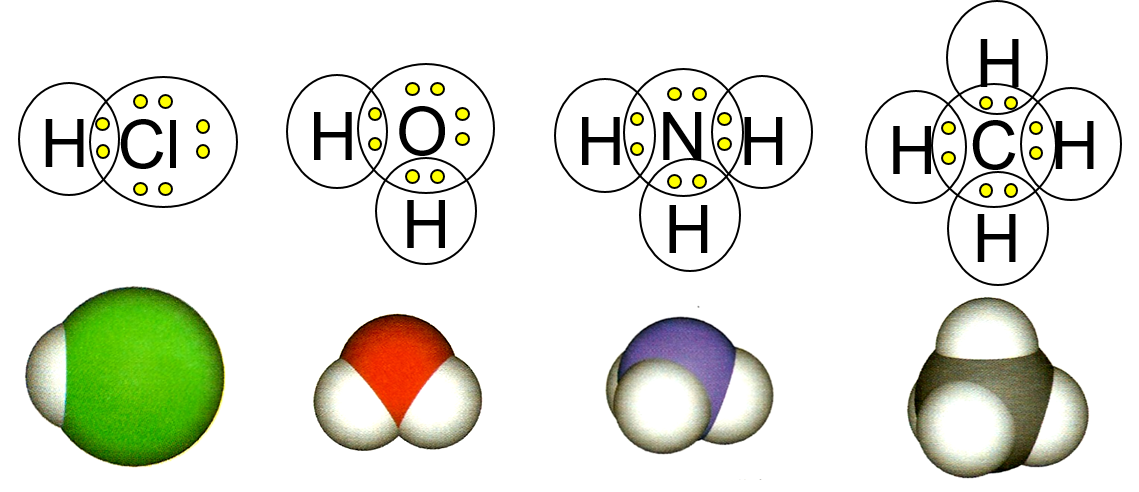
The outermost electrons of different atoms can interact with each other in order to form chemical bonds between the atoms. Covalent bonds are formed when electrons are shared between the two atoms. If the electrons are shared equally, the bond is a nonpolar covalent bond. If the electrons are shared unequally, the bond is a polar covalent bond.
Ionic bonds are formed when electrons are transferred from one atom to another. This results in the formation of a compound known as a salt. For example, table salt (NaCl) is made when sodium loses an electron to chlorine, forming an ionic bond.
Watch the video below for more information about bonding.
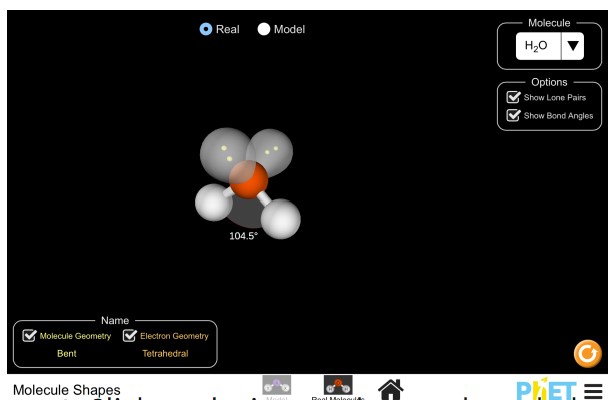
Practice: Understanding Chemistry in 3D
Use the simulation below to explore 3D models of water, methane, ammonia, and carbon dioxide.
- Click on the image above to launch the simulation
- Choose “Real Molecules”. The first molecule that comes up will be H2O.
- Click on “Show Bond Angles” to de-select it. You may also de-select “Show Lone Pairs”.
- Drag the atoms to understand the 3-D shape
- Choose CH4 from the drop-down menu of molecules.
- CH4 is methane, the simplest organic molecule.
- Notice the restraints on the physical space occupied by the atoms as you drag the atoms around.
- Remember these bond angles and constraints when we explore organic chemistry.
- Toggling between “Model” and “Real” will illustrate if the position of the atoms are influenced by other factors we don’t see.
- Choose NH3 from the drop-down menu.
- NH3 is ammonia, a polar solvent.
- NH3 is NOT an organic molecule.
- Toggle the lone pairs to see how these electrons play a role in the positioning of atoms.
- Choose CO2 from the drop-down menu.
- Explore this non-organic molecule to visualize the effects of double bonds on geometry.