Table of Contents
Metabolism
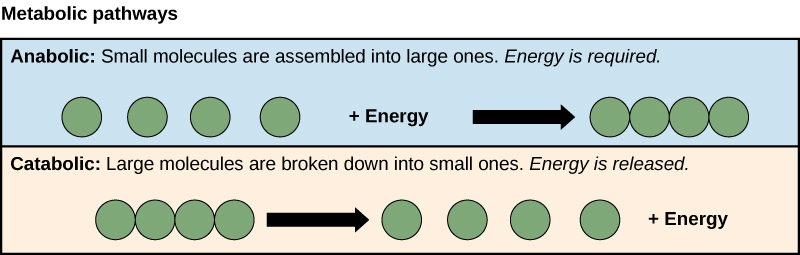
Metabolism consists of the sum total of chemical reactions within cells. They consist of Anabolic (building or synthetic) and Catabolic (degrading) pathways.
Energy released from catabolic reactions are used to fuel anabolic reactions.
Exergonic and endergonic reactions

Activation energy EA
In Biological systems, energy is roughly defined as the capacity to do work. Molecules are held together by electrons. Breaking and building these bonds requires an input of energy. The energy needed to initiate such reactions is referred to as activation energy (EA). Sometimes the necessary energy to initiate a reaction is so great, that it greatly limits the likelihood of the reaction ever occurring. Catalysts are chemicals that take part in facilitating reactions by reducing the energy of activation. If the activation energy is reduced, the likelihood of a reaction occurring is greatly enhanced. In cells, the catalysts are often made of proteins and called enzymes.
The enzyme reduce the EA to facilitate the likelihood that the reaction occurs. The exergonic catabolic reaction breaks complex things down, thus increasing entropy and releasing energy into the system. The endergonic anabolic reactions creates bonds between small molecules to form a large one, thus decreasing entropy and adding energy into the substance.