Table of Contents
What are carbohydrates?
Carbohydrates serve 2 major functions: energy and structure. As energy, they can be simple for fast utilization or complex for storage. Simple sugars are monomers called monosaccharides. These are readily taken into cells and used immediately for energy. The most important monosaccharide is glucose (C6H12O6), since it is the preferred energy source for cells. The conversion of this chemical into cellular energy can be described by the equation below:
C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + energy
Long polymers of carbohydrates are called polysaccharides and are not readily taken into cells for use as energy. These are used often for energy storage. Examples of energy storage molecules are: amylose or starch (plants) and glycogen (animals). Some polysaccharides are so long and complex that they are used for structure like cellulose in the cell walls of plants. Cellulose is very large and practically indigestible, making it unsuitable as a readily available energy source for cells.

Types of simple sugars (or monosaccharides)

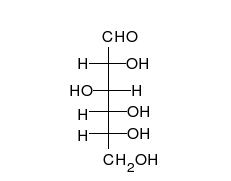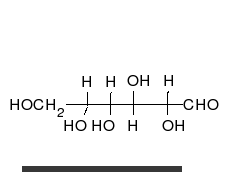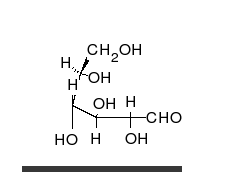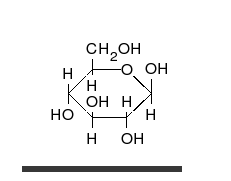
Monosaccharides contain a carbonyl group (C=O). The carbonyl is a source of electrons (the double bond on the oxygen). These electrons can be donated (or lost and oxidized) to reduce another compound (that gains those electrons).
Sugars with an aldehyde group are aldose and sugars with a ketone group are ketose.
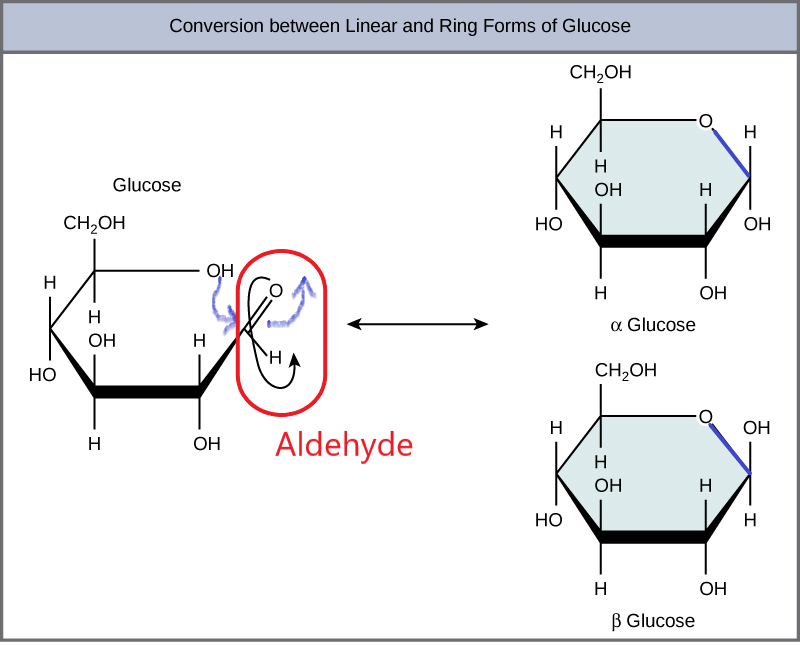
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. This is called isomerization.
Isomerization
Structural polysaccharides
In food, more complex carbohydrates are derived from larger polysaccharides. These larger carbohydrates are fairly insoluble in water. Dietary fiber is name given to indigestible materials in food most often derived from the complex carbohydrates from vegetable material. Some of this material serves the plants as a structural component of the cells and is completely insoluble. Cellulose is the major structural carbohydrate found in plant cell walls. Similarly, animals and fungi have structural carbohydrates that are composed of the indigestible compound called chitin.
Cellulose is a complex carbohydrate of glucose molecules. It is the major structural component of plant cell walls. It’s structural durability is enhanced by intramolecular hydrogen bonds.
In cellulose, glucose monomers are linked by β 1-4 glycosidic bonds that can not be broken by our enzymes.
Chitin is a structural carbohydrate found in animal shells or fungi cell walls. The polymer contains amide groups that differentiates it from other carbohydrates composed of glucose.





