Table of Contents
High surface tension
Surface tension presents as an invisible film that encompasses the surface of water. The attractive forces arising from the intermolecular cohesion holds the surface of water together.
This water strider is not on top of the water because it is light. They have long and slender legs that keep weight evenly distributed on top of water, and have tiny hair that repel water. The water strider has not broken through the surface of the water and is therefore, on top of the water.
Cohesive property
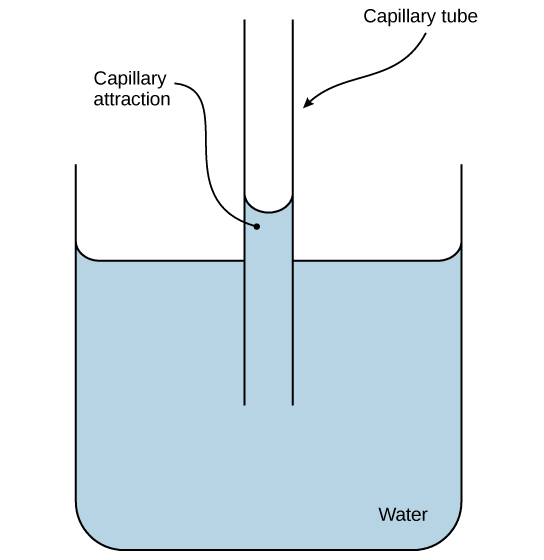
Hydrogen bonds of water can drive adhesion as evidenced by capillary action.
Water travels through our blood vessel the same way.
Evaporative cooling
The H-bonds are responsible for the high boiling point of water (100 degrees C). Evaporation is the removal of water as vapor at a temperature lower than boiling point and is an endothermic reaction. Evaporative cooling describes how the process of liquid evaporation leaves a surface cool. The energy from the heat that is present is transferred to water in sweat where water molecules use this energy to break H-bonds and escape.
High heat capacity
Heat capacity is the amount of energy needed to increase the temperature of a substance. It is measured in calories.
A calorie is defined as the amount of energy required to warm one gram of liquid water by 1ºC. Liquid water requires an abundance of energy to boil because of the hydrogen bonds retain molecules instead of evaporating to release that energy.
In the phase change diagram, you can see that water is liquid in the range of 0 to 100 ºC. The heat energy is used to break the H-bonds. When all the H-bonds are broken, heat is transfer to molecules and change of phase occurs. 80 calories are needed to melt ice cubes and 540 calories are needed to boil water.
Density of ice and water
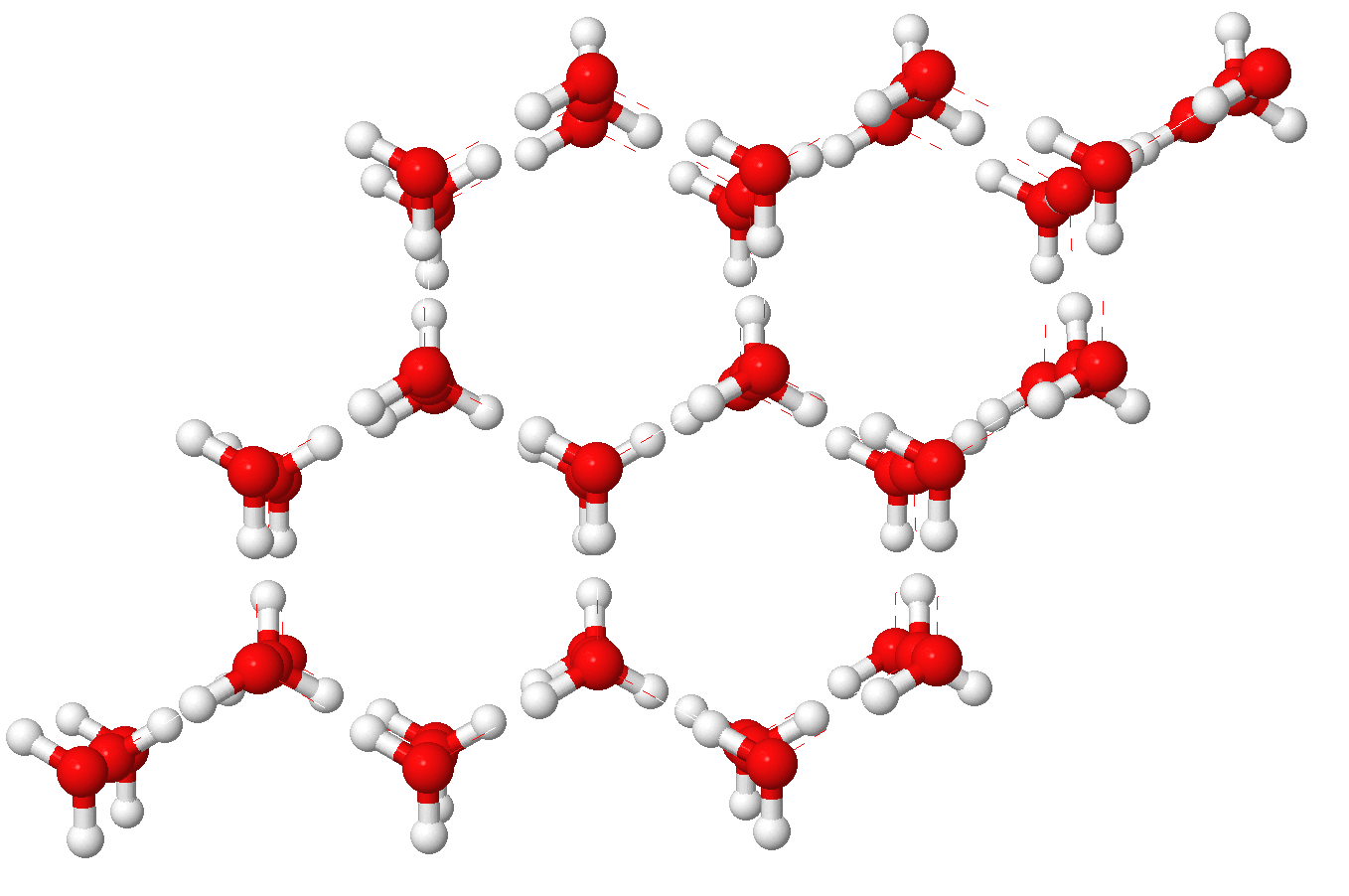
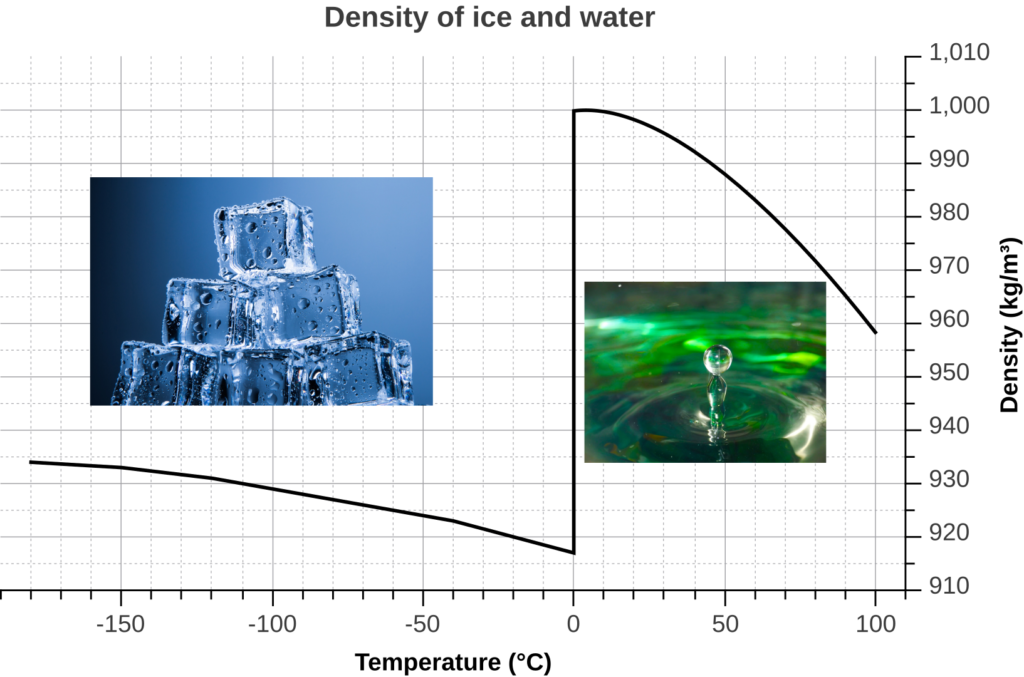

When water cools down to 0ºC, the water molecules move slowly and each molecule makes 4 H-bonds with surrounding water molecules to form an ice lattice. There is more space between water molecules that in liquid water, and there are less water molecules per volume unit. Therefore the density of ice is lower than the density of liquid water.
The curve shows that the density of frozen water is less than the density of liquid water. The density of water is the highest at 4ºC and equals 1kg/m3 or 1g/cm3.
Snow flakes made of water droplets crystalized around air particles form slowly while falling on the ground.








