Click on the picture to go to simulation and choose the Atom button.
Slide 2 protons in the center and 2 electrons in the innermost electron shell so the element is neutral (no net charge). Add 2 neutrons in the center next to the protons. You get Helium which atomic number is 2 and mass number 4.
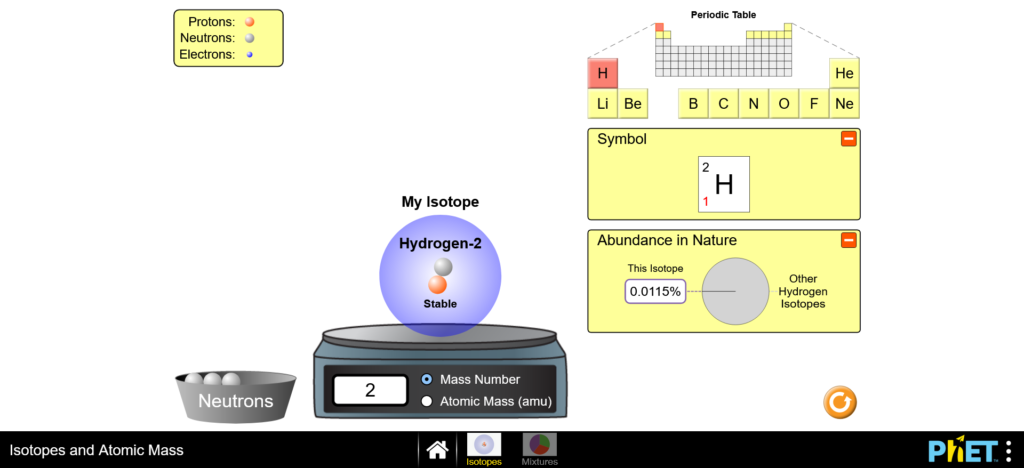
Hydrogen-1 is the most abundant isotope of hydrogen (99.9885%) and has one proton and one electron. The mass number of Hydrogen-1 is 1. Hydrogen-2 or deuterium is an isotope of hydrogen since it has one proton (and one electron). It is an isotope of hydrogen because it has 1 neutron as well, hence its mass number of 2. Hydrogen-2 has a natural abundance of 0.0115%. It is a stable isotope.