Contents
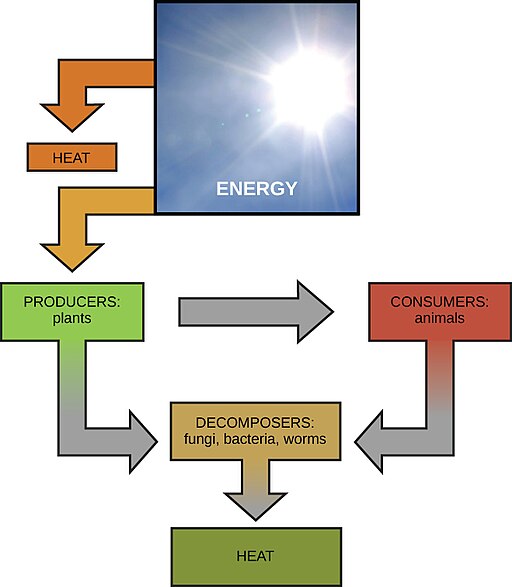
Energy Cycling

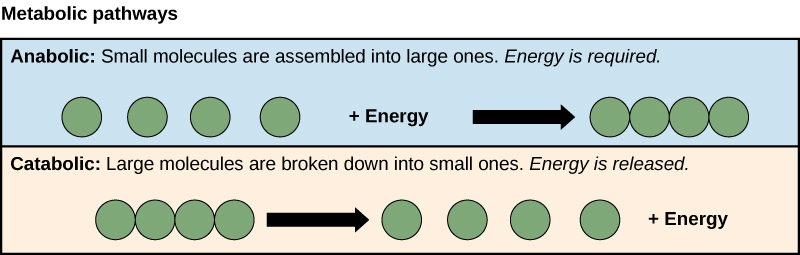
Gibbs Free Energy
Energy and catalysts

In Biological systems, energy is roughly defined as the capacity to do work. Molecules are held together by electrons. Breaking and building these bonds requires an input of energy. The energy needed to initiate such reactions is referred to as activation energy (EA). Sometimes the necessary energy to initiate a reaction is so great, that it greatly limits the likelihood of the reaction ever occurring. Catalysts are chemicals that take part in facilitating reactions by reducing the energy of activation. If the activation energy is reduced, the likelihood of a reaction occurring is greatly enhanced. In cells, the catalysts are often made of proteins and called enzymes.
![By Originally uploaded by Jerry Crimson Mann, vectorized by Tutmosis, corrected by Fvasconcellos (en:Image:Activation2.png) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons reaction coordinates](https://openlab.citytech.cuny.edu/bio-oer/files/2015/09/Activation2_updated.svg_.png)
Enzymes
Reactants in enzymatic reactions are called substrates. They have an imperfect fit to a binding domain of the enzyme called the active site. Substrate binding to this active site induces a change in the shape of the protein that coordinates the substrate into a transition state that will reduce the amount of EA required for the reaction to go to completion. The induced fit of the protein also aids in coordinating other cofactors or coenzymes that will aid in the reaction.

The reaction follows the standard flow where the Enzyme (E) and the Substrate (S) interact to form an Enzyme-Substrate Complex (ES). The ES then dissociates into Enzyme and the resultant Product (P)
E + S ⇒ ES ⇒ E + P
The induced fit of the enzyme-substrate complex coordinates the transition state to facilitate the reaction. This induced fit occurs through non-covalent means that result in a tugging on the molecules (an application of energy) while molecules are coaxed into the reactions.

Cellular Respiration
Glycolysis
The Preparatory Reaction
In the presence of O2, aerobic organisms will use a reaction of pyruvate decarboxylation in the cytosol. This reaction generates a molecule of Acetyl-CoA from the Coenzyme A which can enter the mitochondria.

When there is an excess of carbohydrates, the Acetyl-CoA is used as a starting point for long-term energy storage in lipid synthesis.
Mitochondria
Mitochondria are the power station of eukaryotic cells. They are derived from a process described by the endosymbiotic theory whereby aerobic prokaryotes were engulfed by a protoeukaryote. In this mutualistic arrangement, the prokaryote detoxified the deadly O2 gas in the environment and used it to fully break down glucose to yield many ATP molecules. Evidence for this theory comes from the independent replication of the mitochondria, the bacterial-like mitochondrial DNA, the bacterial-like mitochondrial ribosomes, the bacterial lipids found in the inner membrane and the eukaryotic nature of the outer membrane. Mitochondria are genomically similar to bacteria of the order Rickettsiales. Some bacteria of this order are still free-living and some are intracellular pathogens.

Aerobic Respiration

Citric Acid Cycle
Electron Transport Chain
The electrons stored by NADH and FADH2 are transferred to proteins called cytochromes that have metal centers for conducting these electrons. In the process of moving these electrons, the cytochromes in this Electron Transport Chains (ETC) power the movement of protons into the intermembrane space. The terminus of these electrons is an O2 molecule that is reduced into 1/2 H2O molecules. This apparent movement of water molecules from the chemical synthesis is termed chemiosmosis.

A channel in the membrane called ATP synthase acts as a gateway for the H+ back into the matrix, but use this motion to convert ADP into ATP.