Contents
Proteins
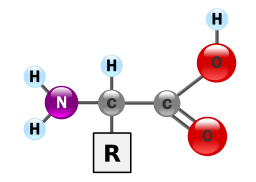 Proteins provide much of the structural and functional capacity of cells. Proteins are composed of monomers called amino acids. Amino Acids are hydrocarbons that have an amino group (-NH2) and an acidic carboxyl group (-COOH).The R group represents a hydrocarbon chain with a modification that alters the properties of the amino acid. 20 universal amino acids are used to construct proteins. The variation in functional groups along the amino acid chain gives rise to the functional diversity of proteins.
Proteins provide much of the structural and functional capacity of cells. Proteins are composed of monomers called amino acids. Amino Acids are hydrocarbons that have an amino group (-NH2) and an acidic carboxyl group (-COOH).The R group represents a hydrocarbon chain with a modification that alters the properties of the amino acid. 20 universal amino acids are used to construct proteins. The variation in functional groups along the amino acid chain gives rise to the functional diversity of proteins.

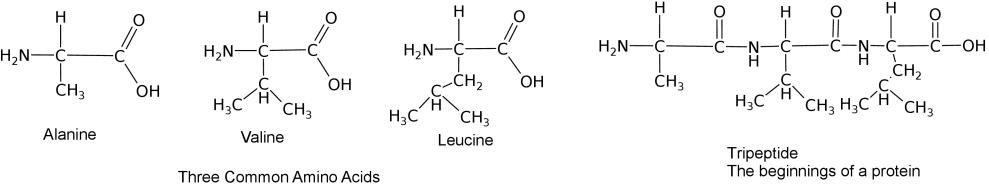
How amino acids interact with each other and the environment
Use the following simulation to test how a polypeptide chain with fold based on the type of solution it is in and the composition of the amino acids.
- Protein Folding Simulation (CC BY 4.0 Concord Consortium)
Protein Function
- Enzymes
- Help “catalyze” chemical reactions
- Structural Proteins
- Give support, strength, flexibility
- Examples: collagen, elastin, keratin
- Storage Proteins
- Storage of amino acids, for building proteins later or energy
- Examples: egg albumin (ovalbumin), Casein (in milk)
- Transport Proteins
- Examples: Hemoglobin (O2), H+ pump
- Signaling Proteins
- Hormonal Proteins and receptor proteins
- Example: Insulin and Insulin receptor
- Motor Proteins
- Actin and myosin perform muscle contraction
- Immune Defense Proteins
- Antibodies help fight infection
Nucleic Acids
Nucleic acids are polymers that were named because they were first described in the nucleus. There are 2 types, deoxyribonucleic acid and ribonucleic acid (DNA & RNA). DNA is the storage vessel for genetic information and RNA largely plays a role in expressing this genetic information.
Their monomers are called nucleotides, which are made up of individual subunits. Nucleotides consist of a 5-Carbon sugar (a pentose), a charged phosphate and a nitrogenous base (Adenine, Guanine, Thymine, Cytosine or Uracil). Each carbon of the pentose has a position designation from 1 through 5. One major difference between DNA and RNA is that DNA contains deoxyribose, and RNA contains ribose. The discriminating feature between these pentoses is at the 2′ position where a hydroxyl group in ribose is substituted with a hydrogen.

The following video illustrates the structure and properties of DNA

DNA is a double helical molecule. Two anti-parallel strands are bound together by hydrogen bonds. Adenine forms 2 H-bonds with Thymine. Guanine forms 3 H-bonds with Cytosine. This AT & GC matching is referred to as complementarity. While the nitrogenous bases are found on the interior of the double helix (like rungs on a ladder), the repeating backbone of pentose sugar and phosphate form the backbone of the molecule. Notice that phosphate has a negative charge. This makes DNA and RNA, overall negatively charged.
ATP
- ATP (adenosine triphosphate) is composed of adenine, ribose, and three phosphates
- In cells, one phosphate bond is hydrolyzed – Yields:
- The molecule ADP (adenosine diphosphate)
- An inorganic phosphate molecule
- Energy used for work in the cell


Additional Resources
- Fats and Proteins http://www.visionlearning.com/en/library/Biology/2/Fats-and-Proteins/62