Solutions
Water is an excellent solvent of other polar compounds. Table salt (NaCl) ionizes readily in water. The δ– O associate around Na+ while the δ+ H associate with the Cl–. If NaCl is dissolved in H2O, what do you think happens to the intermolecular interactions between water molecules? What do you think would happen to the H-bonds? Would you expect there to be a difference in the surface tension? How do you think this explains the difference of boiling or freezing?


The process can be described in a simplified way as the separation of a water molecule into a hydrogen ion (H+) and a hydroxide ion (OH-)
pH

These ions, OH- and H+, (while very rare) are EXTREMELY important
They are very reactive
They establish acid-base reactions in aqueous solutions
Changes in concentrations of H+ and OH- can drastically affect the chemistry of a cell
Concentrations of H+ and OH- are equal in pure water
In aqueous solutions, adding certain solutes, called acids and bases, modifies the concentrations of H+ and OH-
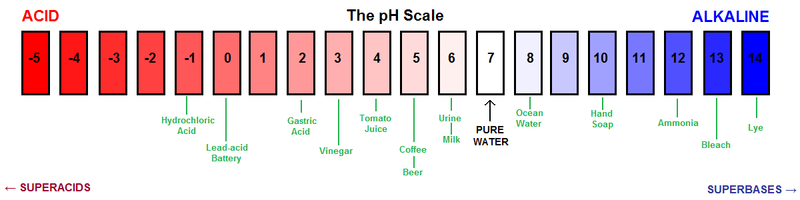
Biologists use something called the pH scale to describe the concentration of these ions a solution
The pH of a solution is dependent on H+ ions:
pH = -log[H +] (in M) AND…
[H+] x [OH-] = 10-14 M (M = mol/L)