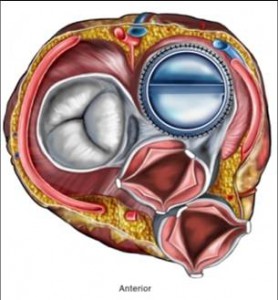
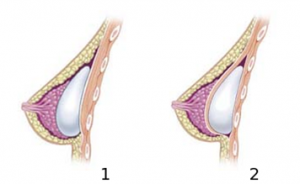
- A medical device is an instrument, apparatus, implant, in vitro reagent, or related article that is used to diagnose, prevent, or treat disease or other conditions, and does not achieve its purposes through chemical action within or on the body (which would make it a drug). Whereas medicinal products (also called pharmaceuticals) achieve their principal action by pharmacological, metabolic or immunological means, medical devices act by other means like physical, mechanical, or thermal means.
Leetyler Dailey's ePortfolio
Building the perfect human being!!!